| Animal Research: |
| Journal of Neuroinflammation, 2020, 17(1). | | The analgesic action of larixyl acetate, a potent TRPC6 inhibitor, in rat neuropathic pain model induced by spared nerve injury.[Reference: WebLink] | Neuropathic pain is a debilitating status that is insusceptible to the existing analgesics. It is important to explore the underlying pathophysiological changes and search for new pharmacological approaches. Transient receptor potential canonical 6 (TRPC6) is a mechanosensitive channel that is expressed by dorsal root ganglia and glial cells. It has been demonstrated that this channel in dorsal root ganglia plays essential roles in the formation of mechanical hyperalgesia in neuropathic pain. Recent pharmacological screening suggests that Larixyl acetate (LA), a main constituent of larch resin, is able to selectively inhibit TRPC6 function. But whether LA is effective in treating neuropathic pain remains unknown. We investigated the efficacy of LA in rat neuropathic pain model, examined its effects on central neuroinflammation, and explored the possible molecular mechanisms by targeting the spinal dorsal horn.
|
|
| Structure Identification: |
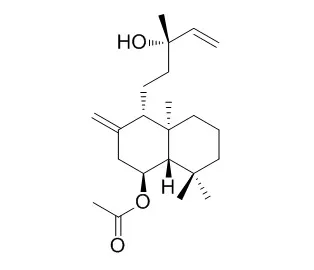
| Journal of Wood science., 01 Oct 2012, 58(5):437-445. | | Accumulation of constitutive diterpenoids in the rhytidome and secondary phloem of the branch bark of Larix gmelinii var. japonica.[Reference: WebLink] | The quantitative compositions of the major constitutive diterpenoids in the rhytidome and secondary phloem of the branch bark of Kuril larch (Larix gmelinii var. japonica) were investigated.
METHODS AND RESULTS:
The eight major diterpenoids were isolated from a diethyl ether extract of the branch bark of L. gmelinii var. japonica and identified as 13-epimanool (1), larixol (2), Larixyl acetate (3), 13-epitorulosyl acetate (4), abietic acid (5), neoabietic acid (6), dehydroabietic acid (7), and isopimaric acid (8). The amount of each diterpenoid was subsequently quantified in both the rhytidome and secondary phloem. All of the diterpenoids were present in both bark tissues, but the amounts were significantly higher in the rhytidome than in the secondary phloem. Developed fusiform resin cavities containing oleoresins were commonly observed in dead secondary phloem captured into the rhytidome of a bark transverse section. The accumulation and distribution of these constitutive diterpenoids in the bark tissues can probably be attributed to terpenoid biosynthesis in the living secondary phloem and the rhytidome formation process.
CONCLUSIONS:
From the viewpoint of constitutive chemical defense in conifers, it is suggested that the tree body may be more effectively defended against natural enemies by the higher amount of diterpenoid amount in the outermost and dead bark tissue, the rhytidome, where the potentially poisonous and easily oxidizable diterpenoids can be safely and stably maintained. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)