| In vitro: |
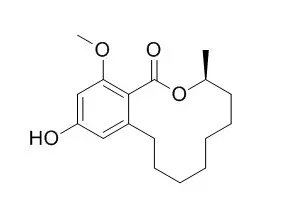
| J Agric Food Chem. 2007 May 16;55(10):4217-21. | | Inhibition of photophosphorylation and electron transport chain in thylakoids by lasiodiplodin, a natural product from Botryosphaeria rhodina.[Pubmed: 17432876] | Four natural products were isolated from the fungus Botryosphaeria rhodina, and their effects on photosynthesis were tested.
METHODS AND RESULTS:
Only Lasiodiplodin (1) inhibited ATP synthesis and electron flow from water to methylviologen; therefore, it acts as a Hill reaction inhibitor in freshly lysed spinach thylakoids. Photosystem I and II and partial reactions as well as ATPase were measured in the presence of 1.
CONCLUSIONS:
Three new different sites of 1 interaction and inhibition were found: one at CF1, the second in the water-splitting enzyme, and the third at the electron-transfer path between P680 and QA; these targets are different from that of the synthetic herbicides present. Electron transport chain inhibition by 1 was corroborated by fluorescence induction kinetics studies. | | Evid Based Complement Alternat Med. 2015;2015:602425. | | Inhibitory Effects of Chemical Compounds Isolated from the Rhizome of Smilax glabra on Nitric Oxide and Tumor Necrosis Factor- α Production in Lipopolysaccharide-Induced RAW264.7 Cell.[Pubmed: 25821492 ] | The rhizome of Smilax glabra has been used for a long time as both food and folk medicine in many countries.
METHODS AND RESULTS:
The present study focused on the active constituents from the rhizome of S. glabra, which possess potential anti-inflammatory activities. As a result, nine known compounds were isolated from the rhizome of S. glabra with the bioassay-guiding, and were identified as syringaresinol (1), Lasiodiplodin (2), de-O-methylLasiodiplodin (3), syringic acid (4), 1,4-bis(4-hydroxy-3,5-dimethoxyphenyl)-2,3-bis(hydroxymethyl)-1,4-butanediol (5), lyoniresinol (6), trans-resveratrol (7), trans-caffeic acid methyl ester (8), and dihydrokaempferol (9). Among these compounds, 2 and 3 were isolated for the first time from S. glabra. In addition, the potential anti-inflammatory activities of the isolated compounds were evaluated in vitro in lipopolysaccharide- (LPS-) induced RAW264.7 cells. Results indicated that 4 and 7 showed significant inhibitory effects on NO production of RAW264.7 cells, and 1, 2, 3, and 5 showed moderate suppression effects on induced NO production. 1, 7, and 5 exhibited high inhibitory effects on TNF-α production, with the IC50 values less than 2.3, 4.4, and 16.6 μM, respectively.
CONCLUSIONS:
These findings strongly suggest that compounds 1, 2, 3, 4, 5, 7, and 9 were the potential anti-inflammatory active compositions of S. glabra. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)