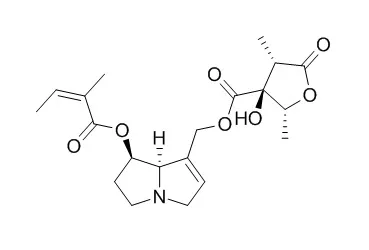
Pyrrolizidine alkaloids (PAs), which are common constituents of hundreds of plant species around the world, have been reported to have cytotoxic, carcinogenic, antineoplastic, and genotoxic activity in vivo and in vitro. The exact mechanism of these biological toxicities is not yet clear.
METHODS AND RESULTS:
The ability of eight PA congeners to inhibit mitosis and induce megalocyte formation in cultured bovine kidney epithelial cells was studied to examine possible structural influences on these endpoints. Representatives of the three PA structural groups, the macrocycles (seneciphylline, senecionine, riddelliine, retrorsine, monocrotaline), open diesters (heliosupine, Latifoline), and a necine base (retronecine), were cocultured for 2 hr with a NADPH-generating system and rat liver S9. Macrocyclic PAs with alpha,beta-unsaturation (seneciphylline, senecionine, riddelliine, retrorsine) showed a dose-dependent inhibition of colony formation at 50, 100, and 300 microM and induction of megalocytosis at 500 microM. Colony growth resumed 3 weeks after removal of PAs at 50 and 100 microM, and normal cellular morphology returned 5 or 6 weeks after removal of PAs at 500 microM. The saturated macrocyclic (monocrotaline) and open diesters (heliosupine, Latifoline), elicited only a slight inhibition of colony formation and had no effect on cellular morphology at 500 microM. The necine base (retronecine) had no effect on either colony formation or cell morphology. Pyrrolic PAs (dehydrosenecionine, dehydromonocrotaline, dehydroretronecine) were more active in inhibition of colony formation than their parent compounds and were potent inducers of abnormal cellular morphology at 500 microM. An N-oxide metabolite, indicine N-oxide, was completely inactive.
CONCLUSIONS:
The results support previous studies showing that there are structural influences on PA-induced cytopathological effects. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)