| Description: |
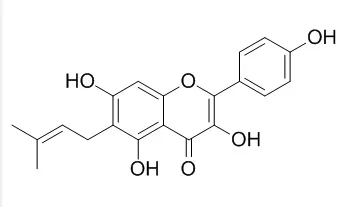
Licoflavonol is a novel natural inhibitor of Salmonella T3SS, could be a promising candidate for novel type of anti-virulence drugs, it exhibits a strong inhibitory effect on the secretion of the SPI-1 effector proteins via regulating the transcription of the SicA/InvF genes, and the transportation of the effector protein SipC. |
| Targets: |
Antifection |
| In vitro: |
| Biochem Biophys Res Commun. 2016 Sep 2;477(4):998-1004. | | Licoflavonol is an inhibitor of the type three secretion system of Salmonella enterica serovar Typhimurium.[Pubmed: 27387231 ] | As an important food-borne human pathogen, Salmonella enterica serovar Typhimurium depends on its type III secretion system (T3SS) as a major virulence factor to cause disease all over the world. The T3SS secretes effector proteins to facilitate invasion into host cells.
METHODS AND RESULTS:
In this study, twenty prenylated flavonoids (1-20) were screened for their anti-T3SS activity, revealing that several analogs exhibited strong inhibitory effects on the secretion of Salmonella pathogenicity island 1 (SPI-1)-associated effector proteins without affecting the growth of bacteria and the secretion of the flagellar protein FliC. Among the flavonoids 1-20, Licoflavonol (20) exhibited a strong inhibitory effect on the secretion of the SPI-1 effector proteins via regulating the transcription of the SicA/InvF genes, and the transportation of the effector protein SipC.
CONCLUSIONS:
In summary, Licoflavonol, a novel natural inhibitor of Salmonella T3SS, could be a promising candidate for novel type of anti-virulence drugs. |
|
| In vivo: |
| J Pharm Biomed Anal. 2015 Nov 10;115:515-22. | | Metabolites identification of bioactive licorice compounds in rats.[Pubmed: 26311472 ] | Licorice (Glycyrrhiza uralensis Fisch.) is one of the most popular herbal medicines worldwide. This study aims to identify the metabolites of seven representative bioactive licorice compounds in rats.
METHODS AND RESULTS:
These compounds include 22β-acetoxyl glycyrrhizin (1), Licoflavonol (2), licoricidin (3), licoisoflavanone (4), isoglycycoumarin (5), semilicoisoflavone B (6), and 3-methoxy-9-hydroxy-pterocarpan (7). After oral administration of 250mg/kg of 1 or 40mg/kg of 2-7 to rats, a total of 16, 43 and 31 metabolites were detected in the plasma, urine and fecal samples, respectively. The metabolites were characterized by HPLC/DAD/ESI-MS(n) and LC/IT-TOF-MS analyses. Particularly, two metabolites of 1 were unambiguously identified by comparing with reference standards, and 22β-acetoxyl glycyrrhizin-6″-methyl ester (1-M2) is a new compound.
CONCLUSIONS:
Compound 1 could be readily hydrolyzed to eliminate the glucuronic acid residue. The phenolic compounds (4-7) mainly undertook phase II metabolism (glucuronidation or sulfation). Most phenolic compounds with an isoprenyl group (chain or cyclized, 2-5) could also undertake hydroxylation reaction. This is the first study on in vivo metabolism of these licorice compounds. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)