| In vitro: |
| Biol Pharm Bull. 2012;35(4):573-81. | | Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing β-D-glucosidase from Bifidobacterium longum H-1.[Pubmed: 22466563] | To understand the role of intestinal microflora in expressing the pharmacological effect of ginsenoside Rb1, the metabolic activity of ginsenoside Rb1 by 148 fecal specimens was measured and its metabolizing β-glucosidase was cloned.
METHODS AND RESULTS:
The average activities for p-nitrophenyl-β-D-glucopyranoside and ginsenoside Rb1 were 0.097±0.059 μmol/min/mg and 0.311±0.118 pmol/min/mg, respectively. These enzyme activities were not different between male and female, or between ages. A gene encoding β-D-glucosidase (BglX) was cloned from Bifidobacterium longum H-1, which transformed ginsenoside Rb1 to compound K. The probe for cloning was synthesized from the genes encoding a β-D-glucosidase of previously reported B. longum DJO10A. The sequences of the cloned gene revealed 2364 bp open reading frame (ORF) encoding a protein containing 787 amino acids (molecular weight of 95 kDa). The gene exhibited 99% homology (identities) to that of B. longum. The cloned gene was expressed under T7 promoter of the expression vector, pET-39b(+), in Escherichia coli BL21(DE3), and the expressed enzyme was purified by using HiTrap immobilized metal affinity chromatography (IMAC) HP. The enzyme potently biotransformed ginsenoside Rb1, loganin, arctiin and arbutin to ginsenoside Rd, Loganetin, arctigenin and hydroquinone, respectively, but was not active in the case of hesperidin, and kakkalide.
CONCLUSIONS:
This is the first report on cloning and expression of β-D-glucosidase from B. longum. Based on these findings, ginsenoside Rb1 may be metabolized to bioactive compound(s) by exo-β-D-glucosidase(s) produced from the intestinal bacteria and its pharmacological effects may be dependent on intestinal bacterial exo-β-D-glucosidase(s) activity. | | Phytochemistry. 2003 Sep;64(2):549-53. | | seco-iridoids from Calycophyllum spruceanum (Rubiaceae).[Pubmed: 12943773] |
METHODS AND RESULTS:
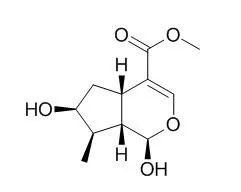
Three seco-iridoids 7-methoxydiderroside, 6'-O-acetyldiderroside and 8-O-tigloyldiderroside, were isolated from the wood bark of Calycophyllum spruceanum together with the known iridoids Loganetin, loganin and the seco-iridoids secoxyloganin, kingiside and diderroside. Their structures were elucidated by means of NMR and MS spectral data analysis. Using NOE correlations and coupling constants, the relative stereochemistry of the new derivatives was established.
CONCLUSIONS:
7-Methoxydiderroside, 6'-O-acetyldiderroside and the known secoxyloganin and diderroside showed in vitro activity against trypomastigote forms of Trypanosoma cruzi, with IC(50) values of 59.0, 90.2, 74,2 and 84.9 microg/mL, respectively and were compared to the standard gentian violet (IC(50) 7.5 microg/ml). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)