| Animal Research: |
| Journal of Biomaterials & Tissue Engineering, 2016 , 6 (6) :427-432. | | Loureirin A Activates Wnt/β-Catenin Pathway to Promote Wound with Follicle Stem Cell-Seeded Tissue-Engineered Skin Healing[Reference: WebLink] | Hair follicle stem cells (FSCs) can participate in the formation of hair follicles and epidermis. FSCs are well known as the ideal seed cells for tissue-engineered skin. Loureirin A, the major constituent of resina draconis, can promote skin wound healing.
METHODS AND RESULTS:
In this study, FSCs and hair follicle fibroblasts were combined with Pelnac® scaffold to construct tissue-engineered skin. The tissue-engineered skin was grafted into full-thickness skin defects of BALB/c-nu mice. Loureirin A was incorporated into the transplanted tissue-engineered skin. On day 3 of post-transplantation, Loureirin A could inhibit inflammatory reaction. On day 7 of post-transplantation, Loureirin A promoted the fibroblasts proliferation to repair dermis, and the newly formed epidermis was thicker than normal epidermis. On day 14 of post-transplantation, Loureirin A promoted the newly formed skin tending to the normal skin. All of these results showed that Loureirin A could promote skin wound repair. Wnt/β-catenin pathway controls natural hair follicle regeneration and epidermis' repairing on skin injuries. In order to validate whether Wnt/β-catenin pathway can be activated by Loureirin A, it was used to stimulate FSCs in vitro. The results displayed that Loureirin A could promote FSCs proliferation and change the cell cycle. Western blot showed that GSK-3β was down-regulated and β-catenin was up-regulated in FSCs by Loureirin A. Simultaneously, downstream genes of Wnt/β-catenin pathway, c-Myc, cyclin D1, Tcf3 and Lef1, also increased.
CONCLUSIONS:
Overall, these results demonstrate that Loureirin A activates Wnt/β-catenin pathway and promotes FSC-seeded tissue-engineered skin to repair skin wound. |
|
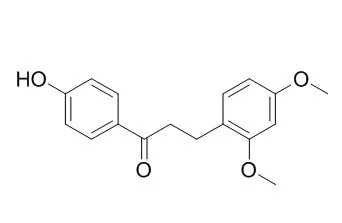
| Structure Identification: |
| Eur J Med Chem. 2015 Mar 26;93:492-500. | | Comparison between loureirin A and cochinchinenin C on the interaction with human serum albumin.[Pubmed: 25734332] |
METHODS AND RESULTS:
The interactions of Loureirin A (LA) and cochinchinenin C (CC) with human serum albumin (HSA) under simulated physiological conditions (pH = 7.4) have been studied with fluorescence, UV-vis absorption spectroscopic method and molecular docking technique. The results indicated that there was a synergistic interaction between LA and CC, and the fluorescence quenching of HSA by LA (or CC) was a combined quenching procedure (dynamic and static quenching). At low compound concentrations, the quenching constants KSV of CC was larger than that of LA, which meant the CC efficacy may be better than that of LA. The negative △H and △S values suggested hydrogen bonds and van der Waals forces played the major role in the binding of LA (or CC) to HSA. The efficiency of energy transfer and distance between the compounds and HSA was calculated.
CONCLUSIONS:
Moreover, the results of synchronous and three-dimensional fluorescence demonstrated that the HSA microenvironment was changed in the presence of LA (or CC). Finally, the binding of LA (or CC) to HSA was modeled by molecular docking, which is in good accordance with the experimental studies. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)