| Structure Identification: |
| J Nat Prod. 2011 Nov 28;74(11):2425-30. | | Alkaloids from the Chinese vine Gnetum montanum.[Pubmed: 22040053 ] | During a high-throughput screening campaign of a prefractionated natural product library, fractions from the Chinese vine Gnetum montanum showed in vitro activity against Pseudomonas aeruginosa wild-type strain, PAO1.
METHODS AND RESULTS:
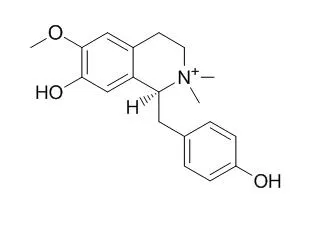
UV-directed isolation of the organic extract from the vine leaves resulted in the purification of the new natural products N-methyllaudanosolinium trifluoroacetate (1), 3'-hydroxy-N,N-dimethylcoclaurinium trifluoroacetate (2), 1,9,10-trihydroxy-2-methoxy-6-methylaporphinium trifluoroacetate (3), and 6a,7-didehydro-1,9,10-trihydroxy-2-methoxy-6-methylaporphinium trifluoroacetate (4). Compound 4 is described here for the first time, and this is the first report of compounds 1-3 as natural products. Compounds 1-3 were found to racemize over time. Starting from commercially available (+)-boldine, through a series of semisynthetic reactions, a mechanism for the racemization of the isolated compounds is proposed.
CONCLUSIONS:
The known natural products (-)-latifolian A (5) and Magnocurarine (6) were also isolated during these studies. The antibacterial activity was explained by the presence of 5, which displayed an IC50 value of 9.8 μM (MIC = 35 μM). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)