| In vitro: |
| Poult Sci. 2013 Jul;92(7):1936-41. | | Efficacy of malic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage.[Pubmed: 23776283] | This work evaluated the effect of Malic acid washing on the growth of Listeria monocytogenes on poultry legs stored at 4°C for 8 d.
METHODS AND RESULTS:
Fresh inoculated chicken legs were dipped into a 1 or 2% Malic acid solution (vol/vol) for 5 min or distilled water (control). Surface pH values, sensorial characteristics (odor, color, texture, and overall appearance) and L. monocytogenes, mesophile, psychrotroph, and Enterobacteriaceae counts were evaluated after treatment (d 0) and after 1, 3, 6, and 8 d of storage at 4°C. Legs washed with 2% Malic acid showed a significant (P < 0.05) inhibitory effect on L. monocytogenes compared with control legs, with a decrease of about 1.66 log units after treatment. Sensory quality was not adversely affected by Malic acid. Treatments with Malic acid reduced bacterial growth and preserved reasonable sensorial quality after storage at 4°C for 6 d.
CONCLUSIONS:
This study demonstrates that, although Malic acid did reduce populations of L. monocytogenes on poultry, it did not completely inactivate the pathogen. The application of Malic acid may be used as an additional hurdle contributing to extend the shelf life of raw poultry. | | Bioresour Technol . 2018 Jun;258:345-353. | | Current advance in biological production of malic acid using wild type and metabolic engineered strains[Pubmed: 29550171] | | Abstract
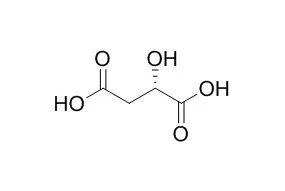
Malic acid (2-hydroxybutanedioic acid) is a four-carbon dicarboxylic acid, which has attracted great interest due to its wide usage as a precursor of many industrially important chemicals in the food, chemicals, and pharmaceutical industries. Several mature routes for Malic acid production have been developed, such as chemical synthesis, enzymatic conversion and biological fermentation. With depletion of fossil fuels and concerns regarding environmental issues, biological production of Malic acid has attracted more attention, which mainly consists of three pathways, namely non-oxidative pathway, oxidative pathway and glyoxylate cycle. In recent decades, metabolic engineering of model strains, and process optimization for Malic acid production have been rapidly developed. Hence, this review comprehensively introduces an overview of Malic acid producers and highlight some of the successful metabolic engineering approaches.
Keywords: Biosynthesis; Carbon dioxide; Metabolic engineering; Renewable resources; l-Malic acid.
Copyright © 2018 Elsevier Ltd. All rights reserved. |
|
| In vivo: |
| J Endourol. 2014 Feb;28(2):229-36. | | Malic acid supplementation increases urinary citrate excretion and urinary pH: implications for the potential treatment of calcium oxalate stone disease.[Pubmed: 24059642] | Raising urinary pH and citrate excretion with alkali citrate therapy has been a widely used treatment in calcium nephrolithiasis. Citrate lowers ionized Ca(+2) concentrations and inhibits calcium salt precipitation. Conservative alternatives containing citrate such as fruit juices have been investigated and recommended. Any compound that induces systemic alkalosis will increase citraturia. Malate, a polycarboxylic anion like citrate, is a potential candidate for chelating Ca(+2) and for inducing systemic alkalinization. We undertook to investigate these possibilities.
METHODS AND RESULTS:
Theoretical modeling of Malic acid's effects on urinary Ca(+2) concentration and supersaturation (SS) of calcium salts was achieved using the speciation program JESS. Malic acid (1200 mg/day) was ingested for 7 days by eight healthy subjects. Urines (24 hours) were collected at baseline and on day 7. They were analyzed for routine lithogenic components, including pH and citrate. Chemical speciation and SS were calculated in both urines.
Modeling showed that complexation between calcium and malate at physiological concentrations of the latter would have no effect on SS. Administration of the supplement induced statistically significant increases in pH and citraturia. The calculated concentration of Ca(+2) and concomitant SS calcium oxalate (CaOx) decreased after supplementation, but these were not statistically significant. SS for the calcium phosphate salts hydroxyapatite and tricalcium phosphate increased significantly as a consequence of the elevation in pH, but values for brushite and octacalcium phosphate did not change significantly.
CONCLUSIONS:
We speculate that consumption of Malic acid induced systemic alkalinization leading to reduced renal tubular reabsorption and metabolism of citrate, and an increase in excretion of the latter. The decrease in SS(CaOx) was caused by enhanced complexation of Ca(+2) by citrate. We conclude that Malic acid supplementation may be useful for conservative treatment of calcium renal stone disease by virtue of its capacity to induce these effects. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)