Natural Products

- ChemFaces is a professional high-purity natural products manufacturer.
- Product Intended Use
- 1. Reference standards
- 2. Pharmacological research
- 3. Inhibitors



| Size /Price /Stock | 10 mM * 1 mL in DMSO / $235.4 / In-stock | Other Packaging | *Packaging according to customer requirements(100uL/well, 200uL/well and more), and Container use Storage Tube With Screw Cap |

| Size /Price /Stock | 10 mM * 100 uL in DMSO / Inquiry / In-stock 10 mM * 1 mL in DMSO / Inquiry / In-stock | Related Libraries |
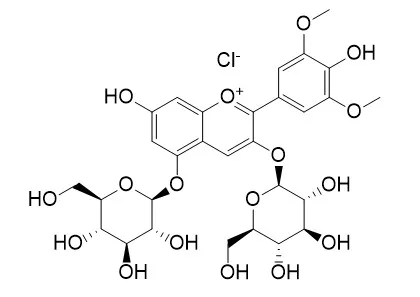
| Description: | Reference standards. |
| Source: | The fruits of Vaccinium myrtillus |
| Solvent: | DMSO, Pyridine, Methanol, Ethanol, etc. |
| Storage: | Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to 24 months(2-8C). Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour. Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com |
| After receiving: | The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling. |
 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4472 mL | 7.2359 mL | 14.4718 mL | 28.9436 mL | 36.1795 mL |
| 5 mM | 0.2894 mL | 1.4472 mL | 2.8944 mL | 5.7887 mL | 7.2359 mL |
| 10 mM | 0.1447 mL | 0.7236 mL | 1.4472 mL | 2.8944 mL | 3.6179 mL |
| 50 mM | 0.0289 mL | 0.1447 mL | 0.2894 mL | 0.5789 mL | 0.7236 mL |
| 100 mM | 0.0145 mL | 0.0724 mL | 0.1447 mL | 0.2894 mL | 0.3618 mL |
| Structure Identification: |
|