| In vitro: |
| Lloydia. 1979 Mar-Apr;42(2):159-62. | | (+)Nortrachelogenin, a new pharmacologically active lignan from Wikstroemia indica.[Pubmed: 449625] |
METHODS AND RESULTS:
A new lignan, (+)-Nortrachelogenin (I), and a known compound, daphnoretin were isolated from Wikstroemia indica C.A. Meyer (Thymelaeaceae).
CONCLUSIONS:
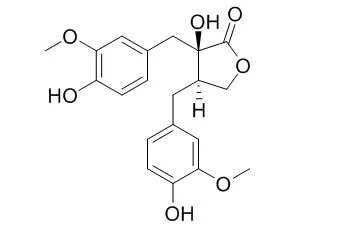
The structure of (+)-Nortrachelogenin was established as 8(R)8'-4,4',8'-trihydroxy-3,3'-dimethoxylignan-olid, (9,9') on the basis of spectroscopic evidence and comparison with its enantiomer, (-)-nortrachelogenin. +-nortrachelogenin(I) showed effects on the central nervous system producing depression in rabbits. | | Planta Med. 2000 Aug;66(6):564-7. | | Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica.[Pubmed: 10985087] |
METHODS AND RESULTS:
With guidance of Pyricularia oryzae bioassay, daphnoretin (1), (+)-Nortrachelogenin (2), genkwanol A (3), wikstrol A (4), wikstrol B (5) and daphnodorin B (6) were isolated from the roots of Wikstroemia indica. Compounds 1-6 induced morphological deformation of P. oryzae mycelia with MMDC values of 68.4 +/- 1.3, 31.3 +/- 1.8, 45.8 +/- 0.5, 70.1 +/- 2.4, 52.3 +/- 0.9 and 73.7 +/- 1.6 microM, respectively.
Compounds 3-6 showed moderate activity against microtubule polymerization with IC50 values of 112 +/- 4, 131 +/- 3, 184 +/- 6 and 142 +/- 2 microM in vitro, respectively.
CONCLUSIONS:
Compounds 2, 3, 5 and 6 were moderately active against HIV-1 in vitro. The findings of bioactivity of 1-6 support the antifungus, antimitosis and anti-HIV-1 uses for W. indica roots. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)