| In vitro: |
| Phytochemistry. 1999 Nov;52(6):1095-9. | | Anti-plasmodial sesquiterpenoids from the African Reneilmia cincinnata.[Pubmed: 10643672] |
METHODS AND RESULTS:
A new isodaucane sesquiterpenoid, 6,7,10-trihydoxyisodaucane, was isolated from the fruits of Reneilmia cincinnata, together with the known sesquiterpenoids oplodiol, Oplopanone, 5E,10(14)-germacradien-1 beta, 4 beta-diol, 1(10)E,5E-germacradien-4 alpha-ol and eudesman-1,4,7-triol. A large amount of 5-hydroxy-3,7,4'-trimethoxyflavone was also isolated. Their structures were established by NMR techniques using 1D and 2D experiments.
CONCLUSIONS:
Three of the known sesquirernenoids exhibited noteworthy anti-plasmodial activity against Plasmodium falciparum strains. | | Nat Prod Res. 2012;26(9):850-8. | | A new sesquiterpenoid from the rhizomes of Homalomena sagittifolia.[Pubmed: 21999629] | A new sesquiterpenoid, 1α,4β,7β-eudesmanetriol (1), was isolated together with the known compounds 1β,4β,7β-eudesmanetriol (2) and Oplopanone (3) from the rhizomes of Homalomena sagittifolia.
METHODS AND RESULTS:
The structures of these compounds were determined by extensive spectral analyses. The compounds 1 and 2 inhibited growth of Pseudomonas stutzeri with a MIC value of 117 µM when evaluated for antibacterial activity using the minimum concentration assay. Both these compounds showed remarkable activities against acetylcholinesterase enzyme with IC(50) values ranging between 25 and 26 µM.
CONCLUSIONS:
The isolation of these sesquiterpenoids and their biological activities observed in this study support the reported traditional uses of H. sagittifolia for the treatment of microbial related diseases and central nervous system disorders. |
|
| In vivo: |
| Arch Pharm Res. 2010 Sep;33(9):1317-23. | | Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo.[Pubmed: 20945129] | Five compounds previously known structures, scopoletin (1), northalifoline (2), stigmast-4-en-3-one (3), tiliroside (4), and Oplopanone (5) were obtained from the flower buds of Magnolia fargesii using chromatographic separation methods.
METHODS AND RESULTS:
The structures of 1-5 were identified by the interpretation of their spectroscopic data including 1D- and 2D-NMR as well as by comparison with reported values. Three compounds 1-3 were found from M. fargesii for the first time in this study. All the isolates (1-5) were subjected to in vitro bioassays to evaluate the inhibitory activity on advanced glycation end products formation and rat lens aldose reductase (RLAR). Compound 1 showed a remarkable inhibitory activity on advanced glycation end products formation with IC(50) value of 2.93 μM (aminoguanidine: 961 μM), and showed a significant RLAR inhibitory activity with IC(50) value of 22.5 μM (3.3-tetramethyleneglutaric acid: 28.7 μM). Compound 4 exhibited potent inhibitory activity against RLAR (IC(50) = 14.9 μM). In the further experiment ex vivo, cataractogenesis of rat lenses induced with xylose was significantly inhibited by compound 1 treatment. |
|


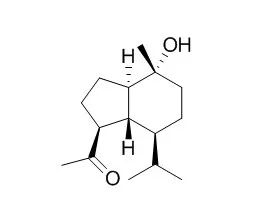



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)