| Animal Research: |
| Planta Medica, 2016,33(3-04):261–267. | | Peltatoside Isolated from Annona crassiflora Induces Peripheral Antinociception by Activation of the Cannabinoid System.[Reference: WebLink] | Peltatoside is a natural compound isolated from leaves of Annona crassiflora Mart., a plant widely used in folk medicine. This substance is an analogue of quercetin, a flavonoid extensively studied because of its diverse biological activities, including analgesic effects. Besides, a previous study suggested, by computer structure analyses, a possible quercetin-CB1 cannabinoid receptor interaction. Thus, the aim of this work was to assess the antinociceptive effect of Peltatoside and analyze the cannabinoid system involvement in this action.
METHODS AND RESULTS:
The mouse paw pressure test was used and hyperalgesia was induced by intraplantar injection of carrageenan (200 µg/paw). All used drugs were administered by intraplantar administration in Swiss male mice (n = 6). Peltatoside (100 µg/paw) elicited a local inhibition of hyperalgesia. The peripheral antinociceptive action of Peltatoside was antagonized by the CB1 cannabinoid antagonist AM251 (160 µg/paw), but not by CB2 cannabinoid antagonist AM630 (100 µg/paw). In order to assess the role of endocannabinoids in this peripheral antinociceptive effect, we used (i) [5Z,8Z,11Z,14Z]-5,8,11,14-eicosatetraenyl-methyl ester phosphonofluoridic acid, an inhibitor of anandamide amidase; (ii) JZL184, an inhibitor for monoacylglycerol lipase, the primary enzyme responsible for degrading the endocannabinoid 2-arachidonoylglycerol; and (iii) VDM11, an endocannabinoid reuptake inhibitor. MAFP, JZL184, and VDM11 did not induce antinociception, respectively, at the doses 0.5, 3.8, and 2.5 µg/paw, however, these three drugs were able to potentiate the peripheral antinociceptive effect of Peltatoside at an intermediary dose (50 µg/paw).
CONCLUSIONS:
Our results suggest that this natural substance is capable of inducing analgesia through the activation of peripheral CB1 receptors, involving endocannabinoids in this process. |
|
| Structure Identification: |
| Fitoterapia, 17 Feb 2013, 86:78-83. | | Antioxidative polyphenols from Nigerian mistletoe Loranthus micranthus (Linn.) parasitizing on Hevea brasiliensis.[Reference: WebLink] |
METHODS AND RESULTS:
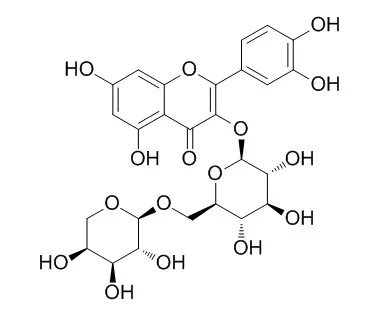
Two new phenolic glycosides, linamarin gallate (1) and walsuraside B (2), together with nine known compounds, catechin (3), epicatechin (4), epicatechin 3-O-gallate (5), epicatechin 3-O-(3-O-methyl)gallate (6), epicatechin 3-O-(3,5-O-dimethyl)gallate (7), epicatechin 3-O-(3,4,5-O-trimethyl)gallate (8), quercetin 3-O-β-d-glucopyranoside (9), rutin (10), and Peltatoside (11), were isolated from the leafy twigs of Nigerian mistletoe Loranthus micranthus (Linn.) parasitic on Hevea brasiliensis. Compound 1 was characterized as an unusual cyanogenic glycoside, while compound 8 was isolated for the first time from a natural source. This is the first report of a cyanogenic glycoside from mistletoes. The structures of the new compounds were unambiguously elucidated by 1D ((1)H, (13)C), 2D NMR (COSY, HSQC, and HMBC) and by mass spectroscopy.
CONCLUSIONS:
The antioxidant activities of the isolated compounds (1-11) were evaluated using the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)