| In vitro: |
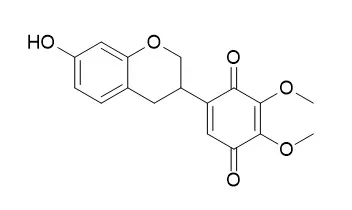
| Planta Med. 2015 Aug;81(12-13):1128-32. | | Antiplasmodial Isoflavanes and Pterocarpans from Apoplanesia paniculata.[Pubmed: 26018916] |
METHODS AND RESULTS:
Bioassay-guided fractionation of an EtOH extract of the roots of the plant Apoplanesia paniculata (Fabaceae) led to the isolation of the three known compounds amorphaquinone (1), Pendulone (2), and melilotocarpan C (3), and the two new pterocarpans 4 and 5.
CONCLUSIONS:
Compounds 1 and 2 exhibited good antiplasmodial activity with IC50 values of 5.7 ± 1.5 and 7.0 ± 0.8 µM, respectively. Compound 3 exhibited weak antiplasmodial activity (41.8 ± 5.2 µM), while compounds 4 and 5 were inactive. Compound 6 was synthesized to confirm the structure of 5, and it showed enhanced antiplasmodial activity (15.8 ± 1.4 µM) compared to its analogues 3-5. | | Nat Prod Commun. 2011 Nov;6(11):1645-50. | | Antiparasitic and antimicrobial isoflavanquinones from Abrus schimperi.[Pubmed: 22224279] |
METHODS AND RESULTS:
The EtOH extract of Abrus schimperi (Fabaceae), collected in Kenya, demonstrated significant activity against Leishmania donovani promastigotes with IC50 value of 3.6 microg/mL.
Bioassay-guided fractionation of CHCl3 fraction using Centrifugal Preparative TLC afforded two antiparasitic isoflavanquinones, namely amorphaquinone (1) and Pendulone (2). They displayed IC50 values of 0.63 microg/mL and 0.43 microg/mL, respectively, against L. donovani promastigotes. Both the compounds were also evaluated against L. donovani axenic amastigotes and amastigotes in THPI macrophage cultures. In addition, compounds 1 and 2 showed antiplasmodial activity against Plasmodium falciparum D6 and W2 strains, while 2 displayed antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus (each IC50 1.44 microg/mL).
CONCLUSIONS:
The 1H and 13C data of 1, not fully assigned previously, were unambiguously assigned using 1D and 2D NMR HMBC and HMQC experiments. In addition, the absolute stereochemistry of the isolated compounds 1 and 2 was revised as C-(3S) based on Circular Dichroism experiments. This appears to be the first report of amorphaquinone (1) and Pendulone (2) from the genus Abrus. | | Chem Pharm Bull (Tokyo). 2006 Jun;54(6):915-7. | | In vitro leishmanicidal constituents of Millettia pendula.[Pubmed: 16755071] | The in vitro leishmanicidal constituents of Millettia pendula were examined.
METHODS AND RESULTS:
Two new compounds, 1 (millettilone A) and 2 (millettilone B), were isolated from the methanol extract of M. pendula, together with six known compounds: 3R-claussequinone (3), Pendulone (4), secundiflorol I (5), 3,8-dihydroxy-9-methoxypterocarpan (6), 3,10-dihydroxy-7,9-dimethoxypterocarpan (7), and formononetin (8). Among these, Pendulone showed the most potent leishmanicidal activity. Compound 2 was found to be a purple pigment in this heartwood. Their chemical structures were elucidated using spectral methods. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)