| Steroids. 2014 Oct;88:60-5. |
| Unprecedent aminophysalin from Physalis angulata.[Pubmed: 24973634 ] |
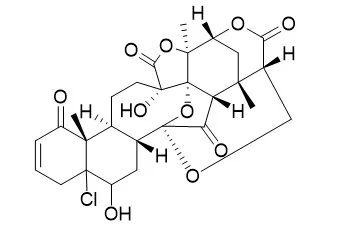
METHODS AND RESULTS:
The 95% ethanol extract of the whole plant of Physalis angulata Linn. afforded one new skeletal physalin named aminophysalin A (1) and one new naturally occurring 5β-hydroxy-6a-chloro-5,6-dihydrophysalin B (2), together with five known physalins (3-7). Their structures were elucidated through MS, IR, NMR spectroscopy analyses and X-ray crystallography. Aminophysalin A (1) had an absolutely unusual structural feature in the chemistry of physalins with a nitrogen atom. Compounds 1-7 were evaluated for quinone reductase activities in hepa 1c1c7 cells.
CONCLUSIONS:
Physalin H (6) showed strong quinone reductase induction activity with IR (Induction ratio, QR induction activity) value of 3.74±0.02, using 4-bromoflavone as a positive control substance (2.17±0.01, 10 μg/mL), while compounds 1, 2, 3, 5 showed weak quinone reductase induction activity. |
| Beilstein J Org Chem. 2014 Jan 13;10:134-40. |
| Physalin H from Solanum nigrum as an Hh signaling inhibitor blocks GLI1-DNA-complex formation.[Pubmed: 24454566 ] |
Hedgehog (Hh) signaling plays an important role in embryonic development, cell maintenance and cell proliferation. Moreover, Hh signaling contributes to the growth of cancer cells. Physalins are highly oxidized natural products with a complex structure.
METHODS AND RESULTS:
Physalins (1-7) were isolated from Solanum nigrum (Solanaceae) collected in Bangladesh by using our cell-based assay. The isolated physalins included the previously reported Hh inhibitors 5 and 6. Compounds 1 and 4 showed strong inhibition of GLI1 transcriptional activity, and exhibited cytotoxicity against cancer cell lines with an aberrant activation of Hh signaling.
Compound 1 inhibited the production of the Hh-related proteins patched (PTCH) and BCL2.
CONCLUSIONS:
Analysis of the structures of different physalins showed that the left part of the physalins was important for Hh inhibitory activity. Interestingly, Physalin H (1) disrupted GLI1 binding to its DNA binding domain, while the weak inhibitor physalin G (2) did not show inhibition of GLI1-DNA complex formation. |
| Chem Biodivers. 2005 Sep;2(9):1164-73. |
| Antileishmanial physalins from Physalis minima.[Pubmed: 17193198 ] |
METHODS AND RESULTS:
Three new physalins (1-3) and a new withanolide 7 have been isolated from the whole plant of Physalis minima, along with three known physalins: Physalin H (4), isophysalin B (5), and 5beta,6beta-epoxyphysalin B (6). Their structures were deduced on the basis of in-depth spectroscopic analyses.
CONCLUSIONS:
Compounds 1-6 showed significant in vitro leishmanicidal activities (0.92-19.4 microg/ml) against promastigotes of Leishmania major. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)