| In vitro: |
| Bioscience Biotechnology and Biochemistry, 1997, 61(2):263-266. | | Spice Constituents Scavenging Free Radicals and Inhibiting Pentosidine Formation in a Model System.[Reference: WebLink] | Many antioxidants have been found in spices and herbs, and some of them are well known as strong scavengers of active oxygen radicals. We have isolated active products, which markedly inhibited the formation of malondialdehyde (MDA) from 2-deoxyribose and the hydroxylation of benzoate with the hydroxyl radical, from methanol extracts of allspice and clove.
METHODS AND RESULTS:
Pimentol from allspice, and biflorin and its isomer, abbreviated as clove3, from clove were identified as the active principles. These revealed strong activity as hydroxyl radical scavengers at a concentration of 2.0 μμ. The antioxidative activities in an in vitro model system involving the rabbit erythrocyte membrane ghost were as strong as those of α-tocopherol at 200 μm. Such advanced glycation end products (AGE) as pentosidine are biomarkers of diabetes mellitus, and active oxygens have been suggested to be involved in the formation of AGE.
CONCLUSIONS:
The above-mentioned free radical scavengers effectively inhibited the formation of pentosidine in a model system of Nα-t-butoxycarbonyl-fructoselysine and Nα-t-butoxycarbonyl-arginine. | | Journal of Natural Products, 2000, 63(6):749-752. | | Galloylglucosides from berries of Pimenta dioica.[Reference: WebLink] |
METHODS AND RESULTS:
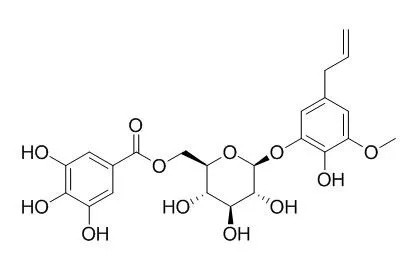
Three new galloylglucosides, (4S)-alpha-terpineol 8-O-beta-D-(6-O-galloyl)glucopyranoside (1); (4R)-alpha-terpineol 8-O-beta-D-(6-O-galloyl)glucopyranoside (2), and 3-(4-hydroxy-3-methoxyphenyl)propane-1,2-diol 2-O-beta-D-(6-O-galloyl)glucopyranoside (3), were isolated from the berries of Pimenta dioica together with three known compounds, gallic acid (4), Pimentol (5), and eugenol 4-O-beta-D-(6-O-galloyl)glucopyranoside (6). The structures of 1-3 were elucidated on the basis of MS and NMR spectral data and enzymatic hydrolysis.
CONCLUSIONS:
These galloylglucosides (1-3, 5, and 6) showed radical-scavenging activity nearly equivalent to that of gallic acid (4) against 1,1-diphenyl-2-picrylhydrazyl radical. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)