| Kinase Assay: |
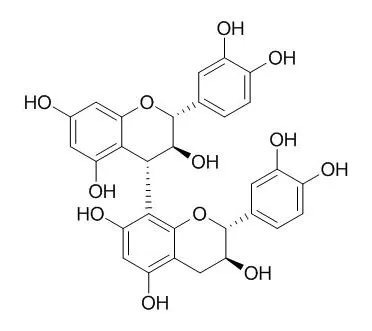
| J Agric Food Chem. 2011 Nov 9;59(21):11794-802. | | Influence of carbohydrates on the interaction of procyanidin B3 with trypsin.[Pubmed: 21950419] | The biological properties of procyanidins, in particular their inhibition of digestive enzymes, have received much attention in the past few years. Dietary carbohydrates are an environmental factor that is known to affect the interaction of procyanidins with proteins. This work aimed at understanding the effect of ionic food carbohydrates (polygalacturonic acid, arabic gum, pectin, and xanthan gum) on the interaction between procyanidins and trypsin.
METHODS AND RESULTS:
Physical-chemical techniques such as saturation transfer difference-NMR (STD-NMR) spectroscopy, fluorescence quenching, and nephelometry were used to evaluate the interaction process. Using STD-NMR, it was possible to identify the binding of Procyanidin B3 to trypsin. The tested carbohydrates prevented the association of Procyanidin B3 and trypsin by a competition mechanism in which the ionic character of carbohydrates and their ability to encapsulate procyanidins seem crucial leading to a reduction in STD signal and light scattering and to a recovery of the proteins intrinsic fluorescence. On the basis of these results, it was possible to grade the carbohydrates in their aggregation inhibition ability: XG > PA > AG ≫ PC.
CONCLUSIONS:
These effects may be relevant since the coingestion of procyanidins and ionic carbohydrates are frequent and furthermore since these might negatively affect the antinutritional properties ascribed to procyanidins in the past. | | Biochem J. 2011 Jan 1;433(1):235-44. | | Procyanidin B3, an inhibitor of histone acetyltransferase, enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor.[Pubmed: 20955177] | Increasing evidence suggests that AR (androgen receptor) acetylation is critical for prostate cancer cell growth.
METHODS AND RESULTS:
In the present study, we identified Pro-B3 (Procyanidin B3) as a specific HAT (histone acetyltransferase) inhibitor. Procyanidin B3 selectively inhibited the activity of HATs, but not other epigenetic enzymes. Procyanidin B3 substantially inhibited the p300-mediated AR acetylation, both in vitro and in vivo. Procyanidin B3 inhibited both p300-dependent and agonist-induced AR transcription. We demonstrate that the p300-mediated AR acetylation is critical for the hormone responsiveness of AR. Interestingly, B3 treatment efficiently enhanced the antagonist activity of flutamide through suppression of p300 HAT activity, demonstrating that relative p300 activity is critical for the antagonist action.
CONCLUSIONS:
Finally, Procyanidin B3 treatment inhibited acetylation-dependent prostate cell proliferation and expression of cell-cycle control genes, subsequently increasing cell death, indicating the functional importance of AR acetylation for prostate cancer cell growth. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)