| In vitro: |
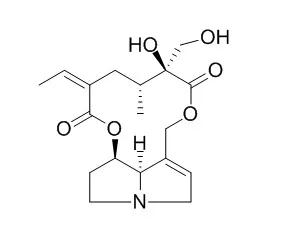
| Toxicology. 2014 Aug 1;322:34-42. | | Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity.[Pubmed: 24799337] | Retrorsine (RTS) is a hepatotoxic pyrrolizidine alkaloid present in plants of the Senecio genus.
The present study is aimed at clarifying the role of organic cation transporters (OCTs) in the liver disposition of Retrorsine, and the coupling of OCT1 and cytochrome P450 (CYP) 3A4 in the hepatotoxicity of RTS.
METHODS AND RESULTS:
MDCK or LLC-PK1 cells stably expressing liver uptake or efflux transporters were used to investigate the interaction of Retrorsine with these transporters. Primary cultured rat hepatocytes (PCRH) and double-transfected MDCK-hOCT1-CYP3A4 cells were used to determine the contribution of OCT1 and CYP3A4 to the toxicity of Retrorsine. The results showed that Retrorsine inhibited the OCT1-mediated 1-methyl-4-phenylpyridinium (MPP(+)) uptake in MDCK-hOCT1 cells with the IC50 of 2.25±0.30μM. The uptake of Retrorsine in MDCK-hOCT1 cells and PCRH was significantly inhibited by OCT1 inhibitors, while hOCT3, human multidrug and toxin extrusion (hMATE) transporter 1, multidrug resistance 1 (MDR1), and breast cancer resistance protein (BCRP) showed weak or no obvious interaction with Retrorsine. The toxic effect of Retrorsine on the PCRH was attenuated by OCT1 inhibitors, quinidine and (+)-tetrahydropalmatine ((+)-THP). Compared to mock cells, MDCK-CYP3A4 cells showed a decrease in viability after being treated with Retrorsine. Furthermore, Retrorsine showed a more severe toxicity in the OCT1/CYP3A4 double-transfected cells compared to all other cells.
CONCLUSIONS:
Our data suggests that OCT1 mediates the liver-specific uptake of Retrorsine, and plays an important role in Retrorsine-induced hepatotoxicity together with CYP3A4. Consequently, the OCT1 inhibitors could be applied to protect the liver from the toxicity of Retrorsine. | | Hepatology. 2013 Mar;57(3):1215-24. | | Contribution of mature hepatocytes to small hepatocyte-like progenitor cells in retrorsine-exposed rats with chimeric livers.[Pubmed: 23080021] | The potential lineage relationship between hepatic oval cells, small hepatocyte-like progenitor cells (SHPCs), and hepatocytes in liver regeneration is debated.
METHODS AND RESULTS:
To test whether mature hepatocytes can give rise to SHPCs, rats with dipeptidyl peptidase IV (DPPIV) chimeric livers, which harbored endogenous DPPIV-deficient hepatocytes and transplanted DPPIV-positive hepatocytes, were subjected to Retrorsine treatment followed by partial hepatectomy (PH). DPPIV-positive hepatocytes comprised about half of the DPPIV chimeric liver mass. Tissues from DPPIV chimeric livers after Retrorsine/PH treatment showed large numbers of SHPC clusters. None of the SHPC clusters were stained positive for DPPIV in any analyzed samples. Furthermore, serial sections stained for gamma-glutamyl-transpeptidase (GGT, a marker of fetal hepatoblasts) and glucose-6-phosphatase (G6Pase, a marker of mature hepatocytes) showed inverse expression of the two enzymes and a staining pattern consistent with a lineage that begins with GGT(+)/G6Pase(-) to GGT(-)/G6Pase(+) within a single SHPC cluster. Using double immunofluorescence staining for markers specific for hepatic oval cells and hepatocytes in serial sections, oval cell proliferations with CK-19(+)/laminin(+) and OV-6(+)/C/EBP-α(-) were shown to extend from periportal areas into the SPHC clusters, differentiating into hepatic lineage by progressive loss of CK-19/laminin expression and appearance of C/EBP-α expression towards the cluster side. Cells in the epithelial cell adhesion molecule (EpCAM(+)) SHPC clusters showed membranous EpCAM(+)/HNF-4α(+) (hepatocyte nuclear factor-4α) staining and were contiguous to the surrounding cytoplasmic EpCAM(+)/HNF-4α(-) ductular oval cells. Extensive elimination of oval cell response by repeated administration of 4,4'-methylenedianiline (DAPM) to Retrorsine-exposed rats impaired the emergence of SHPC clusters.
CONCLUSIONS:
These findings highly suggest the hepatic oval cells but not mature hepatocytes as the origin of SHPC clusters in Retrorsine-exposed rats. | | PLoS One. 2014 May 5;9(5):e95880. | | Disappearance of GFP-positive hepatocytes transplanted into the liver of syngeneic wild-type rats pretreated with retrorsine.[Pubmed: 24796859] | Green fluorescent protein (GFP) is a widely used molecular tag to trace transplanted cells in rodent liver injury models. The differing results from various previously reported studies using GFP could be attributed to the immunogenicity of GFP.
METHODS AND RESULTS:
Hepatocytes were obtained from GFP-expressing transgenic (Tg) Lewis rats and were transplanted into the livers of wild-type Lewis rats after they had undergone a partial hepatectomy. The proliferation of endogenous hepatocytes in recipient rats was inhibited by pretreatment with Retrorsine to enhance the proliferation of the transplanted hepatocytes. Transplantation of wild-type hepatocytes into GFP-Tg rat liver was also performed for comparison.
All biopsy specimens taken seven days after transplantation showed engraftment of transplanted hepatocytes, with the numbers of transplanted hepatocytes increasing until day 14. GFP-positive hepatocytes in wild-type rat livers were decreased by day 28 and could not be detected on day 42, whereas the number of wild-type hepatocytes steadily increased in GFP-Tg rat liver. Histological examination showed degenerative change of GFP-positive hepatocytes and the accumulation of infiltrating cells on day 28. PCR analysis for the GFP transgene suggested that transplanted hepatocytes were eliminated rather than being retained along with the loss of GFP expression. Both modification of the immunological response using tacrolimus and bone marrow transplantation prolonged the survival of GFP-positive hepatocytes. In contrast, host immunization with GFP-positive hepatocytes led to complete loss of GFP-positive hepatocytes by day 14.
CONCLUSIONS:
GFP-positive hepatocytes isolated from GFP-Tg Lewis rats did not survive long term in the livers of Retrorsine-pretreated wild-type Lewis rats. The mechanism underlying this phenomenon most likely involves an immunological reaction against GFP. The influence of GFP immunogenicity on cell transplantation models should be considered in planning in vivo experiments using GFP and in interpreting their results. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)