| Kinase Assay: |
| Medicinal Chemistry Research, 2012 , 21 (6) :722-725. | | Skimmianine, a furoquinoline alkaloid from Zanthoxylum nitidum as a potential acetylcholinesterase inhibitor[Reference: WebLink] | Skimmianine (Skimmianin,1), a newly discovered strong acetylcholinesterase (AChE) inhibitor, along with nine weakly or no active compounds, toddalolactone (2), dictamnine (3), γ-fagarine (4), magnolone (5), (−)-(S)-edulinine (6), zanthodioline (7), edulitine (8), 5,6,7-trimethoxycoumarin (9), and haplopine (10) have been isolated from Zanthoxylum nitidum (Z. nitidum).
METHODS AND RESULTS:
Skimmianine (1) inhibited 50% of AChE activity at the concentrations of 8.6 ± 0.7 μg/ml when the IC50 value of Physostigmine as a standard was 0.013 ± 0.002 μg/ml. Antiacetylcholinesterase activity of Skimmianine (1) was also supported by TLC bioautographic assay.
CONCLUSIONS:
The structure activity relationship on the anti-acetylcholinesterase activity of the quinoline moiety is also discussed in this article. | | J Auton Pharmacol. 1994 Oct;14(5):365-74. | | Skimmianine and related furoquinolines function as antagonists of 5-hydroxytryptamine receptors in animals.[Pubmed: 7829541] | 1. Skimmianine, kokusaginine and confusameline, three furoquinolines extracted from the leaves of Evodia merrillii (Rutaceae), were investigated to characterize their selective effects on subtypes of 5-hydroxytryptamine (5-HT) receptors.
METHODS AND RESULTS:
2. In the isolated membranes of rat cerebrocortex, using [3H]-5-HT and [3H]-ketanserin as radioligands, Skimmianine and the two other furoquinolines displaced radioligand bindings in a concentration-dependent manner. Lower concentrations were required to affect [3H]-ketanserin binding than [3H]-5-HT binding in the order Skimmianine > kokusaginine > confusameline. 3. Furoquinolines inhibited 5-HT-induced contraction mediated by 5-HT2 receptors in the presence of methiothepin in rat isolated aorta. Also, the combination of furoquinolines with ketanserin showed an additive antagonism. 4. These furoquinolines were inactive on the 5-carboxamidotryptamine-induced relaxation of guinea-pig ileum, a 5-HT1-mediated event. However, 5-HT-induced contraction via 5-HT2 receptors was reduced by these furoquinolines in a way similar to that in blood vessels. 5. The failure of these compounds to affect the 5-HT-induced Bezold-Jarisch-like reflex in anaesthetized rats, the major 5-HT3-mediated action, ruled out an action on 5-HT3 receptors.
CONCLUSIONS:
6. The results obtained suggest that three furoquinoline alkaloids may act on 5-HT receptors in animals, more selectively to the 5-HT2 subtype, in the order of Skimmianine > kokusaginine > confusameline. |
|
| Structure Identification: |
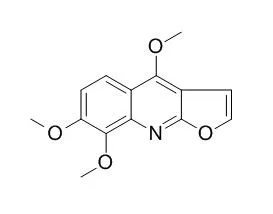
| Acta Crystallographica, 2010 , 59 (10) :o1503-o1505. | | Redetermination of skimmianine: A new inhibitor against the Leishmania APRT enzyme[Reference: WebLink] | The title compound Skimmianin(alternative names 7,8-dimethoxydictammine and 4,7,8-trimethoxyfuro[2,3-b]quinoline), C14H13NO4, is a natural product extracted from Adiscanthus fusciflorus (Rutaceae).
METHODS AND RESULTS:
Our biochemical tests show that it has inhibitory activity against the enzyme adenine phosphoribosyltransferase (APRT) from Leishmania, a tropical parasite causing endemic disease in poor countries. It crystallizes in the centrosymmetric space group P2(1)/c, with one molecule in the asymmetric unit, and has at least two C-H...O intermolecular interactions, leading to the formation of centrosymmetric dimers. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)