| In vitro: |
| Cardiovasc Res . 2011 Nov 1;92(2):307-316. | | Tauroursodeoxycholate (TUDCA) inhibits neointimal hyperplasia by suppression of ERK via PKCα-mediated MKP-1 induction[Pubmed: 21840882] | | Aims: Hyperplasia of vascular smooth muscle cells (VSMCs) after blood vessel injury is one of the major pathophysiological mechanisms associated with neointima. Tauroursodeoxycholate (TUDCA) is a cytoprotective agent in a variety of cells including hepatocytes as well as an inducer of apoptosis in cancer cells. In this study, we investigated whether TUDCA could prevent neointimal hyperplasia by suppressing the growth and migration of VSMCs.
Methods and results: Transporters of TUDCA uptake in human VSMCs (hVSMCs) were analysed by RT-PCR and western blot. A knock-down experiment using specific si-RNA revealed that TUDCA was incorporated into hVSMCs via organic anion transporter 2 (OATP2). TUDCA reduced the viability of hVSMCs, which were mediated by inhibition of extracellular signal-regulated kinase (ERK) by induction of mitogen-activated protein kinase phosphatase-1 (MKP-1) via protein kinase Cα (PKCα). The anti-proliferative effect of TUDCA was reversed by treatment with 7-hydroxystaurosporine, an inhibitor of PKC, and by the knock-down of MKP-1. In addition, TUDCA suppressed hVSMC migration, which was mediated by reduced matrix metalloproteinase-9 (MMP-9) expression by ERK inhibition, as well as reduced viability of hVSMCs. Rats with carotid artery balloon injury received oral administration of TUDCA; this reduced the increase in ERK and MMP-9 caused by balloon injury. TUDCA significantly decreased the ratio of intima to media by reducing proliferation and inducing apoptosis of the VSMCs.
Conclusion: TUDCA inhibits neointimal hyperplasia by reducing proliferation and inducing apoptosis of smooth muscle cells by suppression of ERK via PKCα-mediated MKP-1 induction. | | Cell Signal . 2021 Aug;84:110024. | | Iron overload induces apoptosis of osteoblast cells via eliciting ER stress-mediated mitochondrial dysfunction and p-eIF2α/ATF4/CHOP pathway in vitro[Pubmed: 33901579] | | Iron is an essential element for crucial biological function; whereas excess iron sedimentation impairs the main functions of tissues or organs. Cumulative researches have shown that the disturbances in iron metabolism, especially iron overload is closely concatenating with bone loss. Nevertheless, the specific process of iron overload-induced apoptosis in osteoblasts has not been thoroughly studied. In this study, our purpose is to elucidate the mechanism of osteoblast apoptosis induced by iron overload via the MC3T3-E1 cell line. Ferric ammonium citrate (FAC) was utilized to simulate iron overload conditions in vitro. These results showed that treatment with FAC dose-dependently induced the apoptosis of MC3T3-E1 cells at 48 h, dysfunction of iron metabolism, and increased intracellular reactive oxygen species (ROS) levels. Following, FAC does-dependently caused the calcium dyshomeostasis, decreased the calcium concentration in endoplasmic reticulum (ER), but increased the crosstalk between ER and mitochondria, and calcium concentration in the mitochondria. Moreover, FAC dose-dependently decreased mitochondrial membrane potential (MMP) and enhanced the expression of apoptosis related proteins (Bax, Cyto-C and C-caspase3). We furthermore revealed that FAC treatment activated the ER-mediated cell apoptosis via p-eIF2α/ATF4/CHOP pathway in MC3T3-E1 osteoblasts cells. In addition, pretreatment with the N-acetylcysteine (NAC) or Tauroursodeoxycholate Sodium (TUDC) attenuated cell apoptosis, ROS levels, mitochondria fragmentation and ER stress-related protein expression, and recovered the protein expression related to iron metabolism. In conclusion, our finding suggested that iron overload induced apoptosis via eliciting ER stress, which resulted in mitochondrial dysfunction and activated p-eIF2α/ATF4/CHOP pathway. | | J Hepatol . 2001 Feb;34(2):184-191. | | Effect of tauroursodeoxycholate and S-adenosyl-L-methionine on 17beta-estradiol glucuronide-induced cholestasis[Pubmed: 11281545] | | Background/aims: S-adenosyl-L-methionine (SAMe) and tauroursodeoxycholate (TUDC) exert an additive ameliorating effect on taurolithocholate (TLC)-induced cholestasis. The aims were to investigate the protective effect of SAMe on 17beta-estradiol-glucuronide (17betaEG) cholestasis and to find out whether SAMe and TUDC may exert an additive, ameliorating effect.
Methods: Hepatocyte couplet function was assessed by canalicular vacuolar accumulation (cVA) of cholyllysylfluorescein (CLF). Cells were co-treated with 17betaEG and SAMe, TUDC, or both (protection study), or treated with 17betaEG and then with SAMe, TUDC or both (reversion study) before CLF uptake. Couplets were also co-treated with SAMe and dehydroepiandrosterone (DHEA), a competitive substrate for the sulfotransferase involved in 17betaEG detoxification. The effects of 17betaEG, SAMe and TUDC were also examined on intracellular distribution of F-actin.
Results: Both SAMe and TUDC significantly protected against, and reversed, 17betaEG-induced cholestasis, but their effects were not additive. DHEA abolished the protective effect of SAMe. 17BetaEG did not affect the uptake of CLF into hepatocytes at the concentrations used, and also, it did not affect the intracellular distribution of F-actin.
Conclusions: 17BetaEG does not affect the uptake of CLF into hepatocytes. SAMe and TUDC protect and reverse 17betaEG-induced cholestasis, but without an additive effect. Protection by SAMe may involve facilitating the sulfation of 17betaEG. | | Cell Physiol Biochem . 2015;36(3):866-883. | | Tauroursodeoxycholate Protects Rat Hepatocytes from Bile Acid-Induced Apoptosis via β1-Integrin- and Protein Kinase A-Dependent Mechanisms[Pubmed: 26044599] | | Background/aims: Ursodeoxycholic acid, which in vivo is rapidly converted into its taurine conjugate, is frequently used for the treatment of cholestatic liver disease. Apart from its choleretic effects, tauroursodeoxycholate (TUDC) can protect hepatocytes from bile acid-induced apoptosis, but the mechanisms underlying its anti-apoptotic effects are poorly understood.
Methods: These mechanisms were investigated in perfused rat liver and isolated rat hepatocytes.
Results: It was found that TUDC inhibited the glycochenodeoxycholate (GCDC)-induced activation of the CD95 death receptor at the level of association between CD95 and the epidermal growth factor receptor. This was due to a rapid TUDC-induced β1-integrin-dependent cyclic AMP (cAMP) signal with induction of the dual specificity mitogen-activated protein (MAP) kinase phosphatase 1 (MKP-1), which prevented GCDC-induced phosphorylation of mitogen-activated protein kinase kinase 4 (MKK4) and c-jun-NH2-terminal kinase (JNK) activation. Furthermore, TUDC induced a protein kinase A (PKA)-mediated serine/threonine phosphorylation of the CD95, which was recently identified as an internalization signal for CD95. Furthermore, TUDC inhibited GCDC-induced CD95 targeting to the plasma membrane in a β1-integrin-and PKA-dependent manner. In line with this, the β1-integrin siRNA knockdown in sodium taurocholate cotransporting polypeptide (Ntcp)-transfected HepG2 cells abolished the protective effect of TUDC against GCDC-induced apoptosis.
Conclusion: TUDC exerts its anti-apoptotic effect via a β1-integrin-mediated formation of cAMP, which prevents CD95 activation by hydrophobic bile acids at the levels of JNK activation and CD95 serine/threonine phosphorylation. | | Am J Physiol Gastrointest Liver Physiol . 2004 Jun;286(6):G973-982. | | Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways[Pubmed: 14701718] | | Tauroursodeoxychate (TUDCA) is used for the treatment of cholangiopathies including primary sclerosing cholangitis, which is considered the primary risk factor for cholangiocarcinoma. The effect of TUDCA on cholangiocarcinoma growth is unknown. We evaluated the role of TUDCA in the regulation of growth of the cholangiocarcinoma cell line Mz-ChA-1. TUDCA inhibited the growth of Mz-ChA-1 cells in concentration- and time-dependent manners. TUDCA inhibition of cholangiocarcinoma growth was blocked by BAPTA-AM, an intracellular Ca(2+) concentration ([Ca(2+)](i)) chelator, and H7, a PKC-alpha inhibitor. TUDCA increased [Ca(2+)](i) and membrane translocation of the Ca(2+)-dependent PKC-alpha in Mz-ChA-1 cells. TUDCA inhibited the activity of MAPK, and this inhibitory effect of TUDCA was abrogated by BAPTA-AM and H7. TUDCA did not alter the activity of Raf-1 and B-Raf and the phosphorylation of MAPK p38 and JNK/stress-activated protein kinase. TUDCA inhibits Mz-ChA-1 growth through a signal-transduction pathway involving MAPK p42/44 and PKC-alpha but independent from Raf proteins and MAPK p38 and JNK/stress-activated protein kinases. TUDCA may be important for the treatment of cholangiocarcinoma. |
|
| In vivo: |
| Eur J Vasc Endovasc Surg . 2017 Mar;53(3):337-345. | | Tauroursodeoxycholic Acid Attenuates Angiotensin II Induced Abdominal Aortic Aneurysm Formation in Apolipoprotein E-deficient Mice by Inhibiting Endoplasmic Reticulum Stress[Pubmed: 27889204] | | Objective/background: Abdominal aortic aneurysm (AAA) is characterised by the infiltration of smooth muscle cell (SMC) apoptosis, inflammatory cells, neovascularisation, and degradation of the extracellular matrix. Previous work has shown that endoplasmic reticulum (ER) stress and SMC apoptosis were increased both in a mouse model and human thoracic aortic aneurysm. However, whether the ER stress is activated in AAA formation and whether suppressing ER stress attenuates AAA is unknown.
Methods: Human AAA and control aorta samples were collected. Expression of ER stress chaperones glucose-regulated protein (GRP)-78 and GRP-94 was detected by immunohistochemical staining. The effect of ER stress inhibitor tauroursodeoxycholic acid (TUDCA) on AAA formation in angiotensin (Ang) II induced apolipoprotein E mice was explored. Elastin staining was used to observe the rupture of elastic fragmentation. Immunohistochemistry and Western blot analysis were performed, to detect the protein expression of ER stress chaperones and apoptosis molecules. -/-
Results: There was significant upregulation of GRP-78 and GRP-94 in aneurysmal areas of human AAA and Ang II induced ApoE mice (p < .05). TUDCA significantly attenuated the maximum diameters of abdominal aortas in Ang II induced ApoE mice (p < .05). TUDCA significantly reduced expression of ER stress chaperones and the apoptotic cell numbers (p < .05). Furthermore, TUDCA significantly reduced expression of apoptosis molecules, such as caspase-3, caspase-12, C/EBP homologous protein, c-Jun N-terminal kinase activating transcription factor 4, X-box binding protein, and eukaryotic initiation factor 2α in Ang II induced ApoE mice (p < .05). -/--/--/-
Conclusion: The results suggest that ER stress is involved in human and Ang II induced AAA formation in ApoE mice. TUDCA attenuates Ang II induced AAA formation in ApoE mice by inhibiting ER stress mediated apoptosis. -/--/- | | Eur J Pharmacol . 2001 Jun 1;421(1):55-60. | | Effect of sodium tauroursodeoxycholate on phalloidin-induced cholestasis in rats[Pubmed: 11408049] | | We investigated the therapeutic effect of tauroursodeoxycholate on phalloidin-induced cholestasis in rats. Intrahepatic cholestasis was induced by administration of phalloidin (500 microg/kg, i.p.) for 7 days. From the day of the last phalloidin injection, tauroursodeoxycholate (60-360 micromol/kg) was given intravenously twice a day for 4 days. On the next day after the last tauroursodeoxycholate administration, bile flow, serum biochemical parameters and biliary lipid excretion rates were determined. Tauroursodeoxycholate significantly suppressed the decrease in bile flow and increases in serum alkaline phosphatase, leucine aminopeptidase and glutamic pyruvic transaminase activities, cholesterol, phospholipid and bile acid concentrations observed in phalloidin-induced cholestasis in rats. Furthermore, tauroursodeoxycholate significantly improved the biliary cholesterol and phospholipid excretion rates in phalloidin-induced cholestasis in rats. These results demonstrate the usefulness of tauroursodeoxycholate as a therapeutic agent in intrahepatic cholestasis. |
|


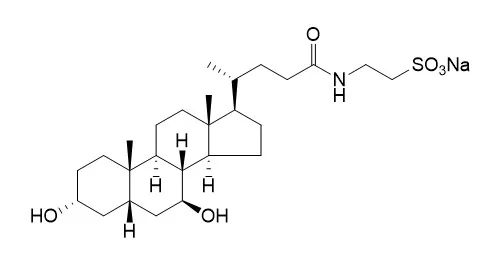



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)