| Food Chem. 2013 Dec 15;141(4):3881-8. |
| ESR studies on the thermal decomposition of trimethylamine oxide to formaldehyde and dimethylamine in jumbo squid (Dosidicus gigas) extract.[Pubmed: 23993561] |
METHODS AND RESULTS:
The effects of ferrous iron, heating temperature and different additives on the decomposition of Trimethylamine oxide (TMAO) to formaldehyde (FA) and dimethylamine (DMA) and generation of free radicals in jumbo squid (Dosidicus gigas) extract during heating were evaluated by electron spin resonance (ESR). The thermal decomposition of Trimethylamine oxide to TMA, DMA and FA and free radical signals was observed in squid extract, whereas no DMA, FA and free radical signals were detected in cod extract or in aqueous Trimethylamine oxide solution in vitro at high temperatures. Significant increase in levels of DMA, FA and radicals intensity were observed in squid extract and Trimethylamine oxide solution in the presence of ferrous iron with increasing temperature. Hydrogen peroxide stimulated the production of DMA, FA and ESR signals in squid extract, while citric acid, trisodium citrate, calcium chloride, tea polyphenols and resveratrol had the opposite effect.
CONCLUSIONS:
Similar ESR spectra of six peaks regarded as amminium radical were detected in the squid extract and Trimethylamine oxide-iron(II) solution, suggesting that the amminium radical was involved in the decomposition of Trimethylamine oxide. |
| Physiol Biochem Zool. 2014 Sep-Oct;87(5):652-62. |
| Coordination of chemical (trimethylamine oxide) and molecular (heat shock protein 70) chaperone responses to heat stress in elasmobranch red blood cells.[Pubmed: 25244377] |
METHODS AND RESULTS:
Using the dogfish (Squalus acanthias) red blood cell (RBC) as a model, we examined whether elasmobranch cells with naturally high concentrations of the chemical chaperone Trimethylamine oxide (TMAO) would induce the molecular chaperone heat shock protein 70 (HSP70) when exposed to an acute thermal stress.
Our hypothesis was that Trimethylamine oxide is itself capable of preventing damage and preserving cellular function during thermal stress and thus that the heat shock response would be inhibited/diminished. We incubated RBCs in vitro with and without physiologically relevant concentrations of Trimethylamine oxide at 13°C and then exposed cells to a 1-h acute heat shock at 24°C. HSP70 protein expression was elevated in dogfish RBCs after the acute heat stress, but this induction was inhibited by extracellular Trimethylamine oxide. Regardless of the presence of Trimethylamine oxide and/or HSP70, we did not observe any cell damage, as indicated by changes in caspase 3/7 activity, protein carbonyls, membrane viability, or levels of ubiquitin. We also saw no change in RBC cell function, as determined by hemoglobin oxygen affinity or carrying capacity, in cells lacking the heat shock response but protected by Trimethylamine oxide.
CONCLUSIONS:
This study demonstrates that there is cellular coordination between chemical and molecular chaperones in response to an acute thermal stress in dogfish RBCs and suggests that Trimethylamine oxide has a thermoprotective role in these cells, thus eliminating the need for a heat shock response. |
| J Gen Microbiol. 1979 Jun;112(2):315-20. |
| Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria.[Pubmed: 479836 ] |
METHODS AND RESULTS:
Trimethylamine oxide (TMAO) stimulated both the anaerobic growth rate and the growth yield of Proteus NTHC 153. The molar growth yield from glucose and pyruvate in tryptone/yeast extract medium doubled in the presence of TMAO, and the organism grew anaerobically on the non-fermentable substrates L-lactate and formate when TMAO was added to the medium.
CONCLUSIONS:
We conclude that TMAO stimulated growth by serving as a terminal electron acceptor in an oxidative phosphorylation process. |


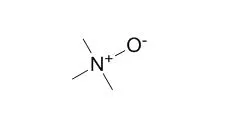



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)