| In vitro: |
| Eur J Med Chem. 2017 Mar 10;128:25-35. | | Design and synthesis of a new series of 3,5-disubstituted isoxazoles active against Trypanosoma cruzi and Leishmania amazonensis.[Pubmed: 28152426 ] | Chagas disease and leishmaniasis are neglected tropical diseases (NTDs) endemic in developing countries. Although there are drugs available for their treatment, efforts on finding new efficacious therapies are continuous.
METHODS AND RESULTS:
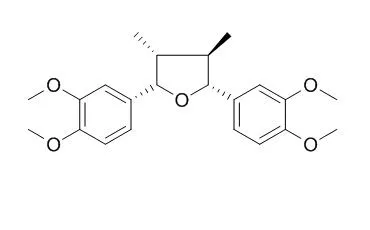
The natural lignans grandisin (1) and Veraguensin (2) show activity against trypomastigote T. cruzi and their scaffold has been used as inspiration to design new derivatives with improved potency and chemical properties. We describe here the planning and microwave-irradiated synthesis of 26 isoxazole derivatives based on the structure of the lignans 1 and 2. In addition, the in vitro evaluation against culture trypomastigotes and intracellular amastigotes of T. cruzi and intracellular amastigotes of L. amazonensis and L. infantum is reported.
CONCLUSIONS:
Among the synthesized derivatives, compounds 17 (IC50 = 5.26 μM for T. cruzi), 29 (IC50 = 1.74 μM for T. cruzi) and 31 (IC50 = 1.13 μM for T. cruzi and IC50 = 5.08 μM for L. amazonensis) were the most active and were also evaluated against recombinant trypanothione reductase of T. cruzi in a preliminary study of their mechanism of action. | | Phytother Res. 2008 Oct;22(10):1307-10. | | In vitro antileishmanial and antimalarial activities of tetrahydrofuran lignans isolated from Nectandra megapotamica (Lauraceae).[Pubmed: 18688887 ] | Seven tetrahydrofuran lignans, isolated from Nectandra megapotamica (Lauraceae), were evaluated for their in vitro antileishmanial and antimalarial activities.
METHODS AND RESULTS:
Among the evaluated compounds, machilin-G (1a) and Veraguensin (2a) showed the highest antileishmanial activities, displaying for both compounds an IC(50) value of 18 microg/mL and an IC(90) value of 36 microg/mL, while galgravin (1b), nectandrin-A (1c), nectandrin-B (1d), calopeptin (2b) and ganshisandrine (3) were inactive against Leishmania donovani. In the antimalarial assay against Plasmodium falciparum, it was observed that calopeptin (2b) displayed moderate activity, with IC(50) values of 3800 ng/mL (D6 clone) and 3900 ng/mL (W2 clone), while the lignans 1a-1d, 2a and 3 were inactive.
CONCLUSIONS:
In order to compare the effect on the parasites with toxicity to mammalian cells, the cytotoxic activity of the isolated compounds were evaluated against the Vero cells, showing that all evaluated tetrahydrofuran lignans exhibited no cytotoxicity at the maximum dose tested. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)