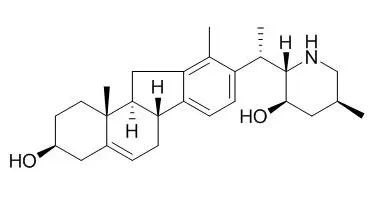
| Description: |
Veratramine exhibits cytotoxic activity against human tumor cell lines A549, PANC-1, SW1990 and NCI-H249. Veratramine shows hypotensive effect, the effect is directly positively correlated with the dosage. |
| In vitro: |
| Phytother Res. 2008 Aug;22(8):1093-6. | | Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum.[Pubmed: 18570211 ] |
METHODS AND RESULTS:
The antitumor activity of six extracts (ethanol extract, petroleum ether fraction, CHCl(3) fraction, ethyl acetate fraction, n-butanol fraction and total alkaloids) from the rhizomes of Veratrum dahuricum, and six compounds (Veratramine (1), jervine (2), germine (3), veramitaline (4), veratrosine (5) and cyclopamine (6)) from the ethanol extract were investigated in vitro. The 12 samples exhibited cytotoxic activity against human tumor cell lines A549, PANC-1, SW1990 and NCI-H249.
CONCLUSIONS:
Among these samples, CHCl(3) fraction, the total alkaloids, compounds 1 and 6 showed higher inhibitory activity, compound 3 selectively exhibited significant cytotoxicity to SW1990 and NCI-H249. |
|
| In vivo: |
| Pharmazie. 2008 Aug;63(8):606-10. | | Hypotensive effect and toxicology of total alkaloids and veratramine from roots and rhizomes of Veratrum nigrum L. in spontaneously hypertensive rats.[Pubmed: 18771011] |
METHODS AND RESULTS:
Total alkaloids (VTA) and Veratramine of Veratrum nigrum L. were tested for hypotensive effect using spontaneously hypertensive rats (SHR). Acute toxicities were also evaluated. There was a dose-dependent reduction in blood pressure and heart rate after a single ingestion (1.0 to 4.0 mg/kg, intragastric administration) of VTA. A single oral ingestion (0.56 to 2.24 mg/kg) of Veratramine, the major component of VTA, dose-dependently decreased blood pressure and heart rate, suggesting that Veratramine was involved in the hypotensive effect of VTA in SHR.
CONCLUSIONS:
The hypotensive effects of VTA and Veratramine are directly positively correlated with the dosage. Side effects were not obvious. | | AAPS J. 2016 Mar;18(2):432-44. | | Gender-Dependent Pharmacokinetics of Veratramine in Rats: In Vivo and In Vitro Evidence.[Pubmed: 26791530] | Veratramine, a major alkaloid from Veratrum nigrum L., has distinct anti-tumor and anti-hypertension effects. Our previous study indicated that Veratramine had severe toxicity toward male rats.
METHODS AND RESULTS:
In order to elucidate the underling mechanism, in vivo pharmacokinetic experiments and in vitro mechanistic studies have been conducted. Veratramine was administrated to male and female rats intravenously via the jugular vein at a dose of 50 μg/kg or orally via gavage at 20 mg/kg. As a result, significant pharmacokinetic differences were observed between male and female rats after oral administration with much lower concentrations of Veratramine and 7-hydroxyl-Veratramine and higher concentrations of Veratramine-3-O-sulfate found in the plasma and urine of female rats. The absolute bioavailability of Veratramine was 0.9% in female rats and 22.5% in male rats. Further experiments of Veratramine on Caco-2 cell monolayer model and in vitro incubation with GI content or rat intestinal subcellular fractions demonstrated that its efficient passive diffusion mediated absorption with minimal intestinal metabolism, suggesting no gender-related difference during its absorption process. When Veratramine was incubated with male or female rat liver microsomes/cytosols, significant male-predominant formation of 7-hydroxyl-Veratramine and female-predominant formation of Veratramine-3-O-sulfate were observed.
CONCLUSIONS:
In conclusion, the significant gender-dependent hepatic metabolism of Veratramine could be the major contributor to its gender-dependent pharmacokinetics. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)