| In vitro: |
| Molecular Pharmacology, 2012, 82(1):134-141. | | The polyphenolic ellagitannin vescalagin acts as a preferential catalytic inhibitor of the α isoform of human DNA topoisomerase II.[Reference: WebLink] |
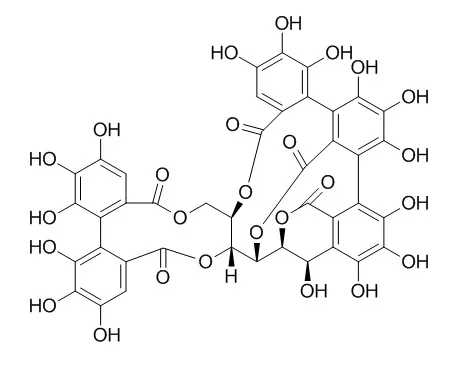
Polyphenolic ellagitannins are natural compounds that are often associated with the therapeutic activity of plant extracts used in traditional medicine. They display cancer-preventing activity in animal models by a mechanism that remains unclear. Potential targets have been proposed, including DNA topoisomerases II (Top2). Top2α and Top2β, the two isoforms of the human Top2, play a crucial role in the regulation of replication, transcription, and chromosome segregation. They are the target of anticancer agents used in the clinic such as anthracyclines (e.g., doxorubicin) or the epipodophyllotoxin etoposide. It was recently shown that the antitumor activity of etoposide was due primarily to the inhibition of Top2α, whereas inhibition of Top2β was responsible for the development of secondary malignancies, pointing to the need for more selective Top2α inhibitors.
METHODS AND RESULTS:
Here, we show that the polyphenolic ellagitannin Vescalagin preferentially inhibits the decatenation activity of Top2α in vitro, by a redox-independent mechanism. In CEM cells, we also show that transient small interfering RNA-mediated down-regulation of Top2α but not of Top2β conferred a resistance to Vescalagin, indicating that the α isoform is a preferential target. We further confirmed that Top2α inhibition was due to a catalytic inhibition of the enzyme because it did not induce DNA double-strand breaks in CEM-treated cells but prevented the formation of Top2α- rather than Top2β-DNA covalent complexes induced by etoposide.
CONCLUSIONS:
To our knowledge, Vescalagin is the first example of a catalytic inhibitor for which cytotoxicity is due, at least in part, to the preferential inhibition of Top2α. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)