METHODS AND RESULTS:
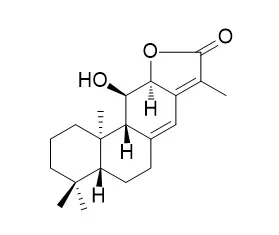
Three new diterpenoids, yuexiandajisu D (1), E (2) and F were isolated from the roots of Euphorbia ebracteolata, along with eight known diterpenoids, jolkinolide B (4), jolkinolide A, ent-11alpha-Hydroxyabieta-8(14),13(15)-dien-16,12alpha-olide (6), ent-(13S)-hydroxyatis-16-ene-3,14-dione, ent-3beta,(13S)-dihydroxyatis-16-en-14-one, ent-3-oxokaurane-16alpha,17-diol, ent-16alpha,17-dihydroxyatisan-3-one and ent-atisane-3beta,16alpha,17-triol. The structures of all compounds were deduced using spectroscopic methods and confirmed for 1 and 2 by single-crystal X-ray diffraction. A biogenetic pathway for the formation of 1 and 2 is proposed briefly. Cytotoxic activities were evaluated against ANA-1, B 16 and Jurkat tumor cells.
CONCLUSIONS:
Jolkinolide B (4) displayed modest activity on ANA-1, B 16 and Jurkat tumor cells with IC50 values 4.46 x 10(-2), 4.48 x 10(-2), 6.47 x 10(-2) microM, and ent-11alpha-Hydroxyabieta-8(14),13(15)-dien-16,12alpha-olide (6) showed significant activity against ANA-1 and Jurkat cells with IC50 values 7.12 x 10(-3) and 1.79 x 10(-2) microM.
Compound 1 was found to be slightly active against ANA-1 cells with an IC50 value 2.88 x 10(-1)microM. Structure-activity relationships of isolated compounds are also discussed. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)