| Structure Identification: |
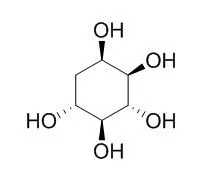
| J Nat Prod. 2007 Mar;70(3):493-7. Epub 2007 Mar 9. | | Synthesis of valiolamine and some precursors for bioactive carbaglycosylamines from (-)-vibo-quercitol produced by biogenesis of myo-inositol.[Pubmed: 17346079] | | A convenient and practical synthesis of valiolamine (4) and its related carbaglycosylamine glycosidase inhibitors from (-)-vibo-Quercitol (13), a compound readily produced by biogenesis of myo-inositol (9), is described. | | Bioorg Med Chem Lett. 2011 Dec 1;21(23):7189-92. | | Transformation of quercitols into 4-methylenecyclohex-5-ene-1,2,3-triol derivatives, precursors for the chemical chaperones N-octyl-4-epi-β-valienamine (NOEV) and N-octyl-β-valienamine (NOV).[Pubmed: 22001090] | | (+)-proto-Quercitol (1) and (-)-vibo-Quercitol (2), both of which could be readily prepared by the bioconversion of myo-inositol, were successfully converted into the corresponding 4-methylenecyclohex-5-ene-1,2,3-triol derivatives. These compounds were demonstrated to be suitable precursors, preserving their configurations, for bioactive carba-aminosugars such as the potent chemical chaperone drug candidates, N-octyl-4-epi-β-valienamine (NOEV, 3) and N-octyl-β-valienamine (NOV, 4). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)