| Structure Identification: |
| Anal Sci. 2010;26(7):743-8. | | MALDI mass spectrometry using 2,4,6-trihydroxyacetophenone and 2,4-dihydroxyacetophenone with cyclodextrins: suppression of matrix-related ions in low-molecular-weight region.[Pubmed: 20631433] |
METHODS AND RESULTS:
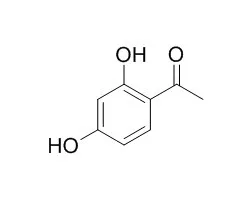
Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry of a model peptide, Substance P (SubP), was carried out using 2,4,6-trihydroxyacetophenone (THAP) and 2,4-Dihydroxyacetophenone (DHAP) with cyclodextrins (cyclodextrin-supported matrix).
It was found that the use of a cyclodextrin-supported matrix simplified the mass spectrum in the low-molecular-weight region. The interaction between THAP/2,4-Dihydroxyacetophenone and cyclodextrin (CD) was studied by UV-vis absorption spectroscopy and the incorporation of matrix molecules into the cyclodextrin cavity was confirmed by (1)H-NMR spectroscopy. 2,4-Dihydroxyacetophenone showed tight incorporation with betaCD (betaCD(2,4-Dihydroxyacetophenone)) rather than THAP and it was found that the matrix-related peaks could be weakened by less than one third of the peak intensity of a protonated analyte. The betaCD(2,4-Dihydroxyacetophenone) matrix was applied to the measurements of two low-molecular-weight compounds; adenosine and adrenaline.
CONCLUSIONS:
It became clear that the cyclic structure of the CD and the host-guest interaction between betaCD and the matrix molecule were important to reduce the matrix-related peaks of THAP and 2,4-Dihydroxyacetophenone. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)