| In vitro: |
| Appl Environ Microbiol. 2012 Sep;78(17):6203-16. | | Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway.[Pubmed: 22752168] |
METHODS AND RESULTS:
Escherichia coli was metabolically engineered by expanding the shikimate pathway to generate strains capable of producing six kinds of aromatic compounds, phenyllactic acid, 4-hydroxyphenyllactic acid, phenylacetic acid, 4-hydroxyphenylacetic acid, 2-phenylethanol, and 2-(4-Hydroxyphenyl)ethanol, which are used in several fields of industries including pharmaceutical, agrochemical, antibiotic, flavor industries, etc.Whereas ipdC and the alcohol dehydrogenase gene (adhC) from Lactobacillus brevis were introduced to generate 2-phenylethanol and 2-(4-Hydroxyphenyl)ethanol producers, respectively. Expression of the respective introduced genes was controlled by the T7 promoter. While generating the 2-phenylethanol and 2-(4-Hydroxyphenyl)ethanol producers, we found that produced phenylacetaldehyde and 4-hydroxyphenylacetaldehyde were automatically reduced to 2-phenylethanol and 2-(4-Hydroxyphenyl)ethanol by endogenous aldehyde reductases in E. coli encoded by the yqhD, yjgB, and yahK genes. Cointroduction and cooverexpression of each gene with ipdC in the phenylalanine and tyrosine overproducers enhanced the production of 2-phenylethanol and 2-(4-Hydroxyphenyl)ethanol from glucose. Introduction of the yahK gene yielded the most efficient production of both aromatic alcohols.
CONCLUSIONS:
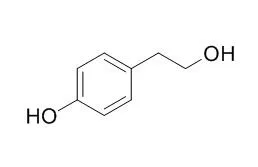
During the production of 2-phenylethanol, 2-(4-Hydroxyphenyl)ethanol, phenylacetic acid, and 4-hydroxyphenylacetic acid, accumulation of some by-products were observed. Deletion of feaB, pheA, and/or tyrA genes from the chromosomes of the constructed strains resulted in increased desired aromatic compounds with decreased by-products. | | Food Chem. 2013 Nov 15;141(2):1147-57. | | Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson's disease.[Pubmed: 23790897 ] | Experimental evidence suggests that tyrosol [2-(4-Hydroxyphenyl)ethanol] exhibits potent protective activities against several pathogeneses.
METHODS AND RESULTS:
In this study, we evaluated the protective effect of tyrosol against 1-methyl-4-phenylpyridinium (MPP(+))-induced CATH.a neuron cell death. Tyrosol dose-dependently protected CATH.a cells from MPP(+)-induced cell death and the protection was more apparent after prolong incubation (48h). The data showed that tyrosol treatment suppressed the reduction of phospho-tyrosine hydroxylase level in CATH.a cells. Further, the compound repressed MPP(+)-induced depletion of mitochondrial membrane potential (Δψm) and thereby maintained intracellular ATP production in the cell. The cellular signalling pathway studies revealed that tyrosol protected CATH.a cells from MPP(+)-induced apoptotic signalling, most likely via activation of PI3K/Akt signalling pathway along with up-regulation of anti-oxidative enzymes (SOD-1 and SOD-2) and DJ-1 protein in the cell.
CONCLUSIONS:
Collectively, present study demonstrates that tyrosol significantly protects dopaminergic neurons from MPP(+)-induced degradation, and reveals potential neuroprotective mechanism of tyrosol. | | Neurochem Int. 2018 Dec;121:140-145. | | Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation.[Pubmed: 30291953 ] | Subsequent inflammation in stroke plays an important role in the damage of neurons in the perilesional area. Therapeutic intervention targeting inflammation may be a promising complementary strategy to current treatments of stroke.
METHODS AND RESULTS:
Here, we explored the possible beneficial effects of tyrosol(2-(4-Hydroxyphenyl)ethanol), a derivative of phenethyl alcohol and natural antioxidant, playing an anti-inflammatory role in astrocyte culture and in vitro oxygen glucose deprivation (OGD) model. MTT, western blot, ELISA and EMSA assays were carried out to investigate cell viability, protein expression level, cytokine expression and NF-κB activity. We found tyrosol protected cultured astrocytes against OGD-induced cell viability loss in MTT test. Meanwhile, tyrosol attenuated the released TNF-α and IL-6 level from astrocyte via regulating Janus N-terminal kinase (JNK). The reduction of cytokines from astrocyte might be due to its inhibition of astrocyte activation and regulation of STAT3 signaling pathway since tyrosol attenuated the expression level of GFAP (glial fibrillary acidic protein) and the phosphorylation of STAT3. Additionally, we demonstrated that tyrosol prevented the degradation of IκBα and the increase of IκBα phosphorylation in astrocytes exposed to OGD, which led to the suppression of NF-κB function during ischemia.
CONCLUSIONS:
Collectively, our results showed that tyrosol may be a promising complementary treatment compound for stroke via modulating the inflammatory response in astrocytes during ischemia. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)