| Description: |
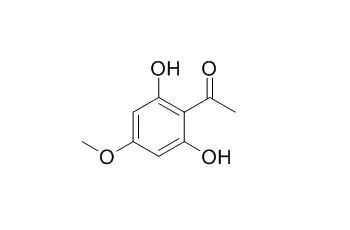
2',6'-Dihydroxy-4'-methoxyacetophenone is a phytoalexin after fungal inoculation with Botrytis cinerea or UV light irradiation, it has spore germination inhibition on Botrytis cinerea and Phomopsis perniciosa, with ED50 values of 45 and 410 uM, respectively. 2',6'-Dihydroxy-4'-methoxyacetophenone exhibits significant antifungal activities. |
| In vitro: |
| Phytochemistry, 1994, 35(2):331-3. | | 2',6'-Dihydroxy-4'-methoxyacetophenone, a phytoalexin from the roots of Sanguisorba minor[Reference: WebLink] |
METHODS AND RESULTS:
The root tissue of Sanguisorba minor produced 2',6'-Dihydroxy-4'-methoxyacetophenone as a phytoalexin after fungal inoculation with Botrytis cinerea or UV light irradiation.
CONCLUSIONS:
The ED50 of this compound on spore germination inhibition was 45 and 410 μM for Botrytis cinerea and Phomopsis perniciosa, respectively. | | Journal of the Japan Wood Research Society, 1997, 43:108-111. | | Antifungal activity of 2′,6′-dihydroxy-4′-methoxyacetophenone and related compounds[Reference: WebLink] |
METHODS AND RESULTS:
We investigated the relationship between the antifungal activity of 2',6'-Dihydroxy-4'-methoxyacetophenone, a component found in the leaves of Rhododendron dauricum L. and of related compounds and their chemical structures, using several common plant pathogenic fungi.
Acetophenones having hydroxyl and/or methoxyl substituents on their aromatic rings showed strong activity against all fungal species tested at a concentration of 500 μml-1. Mono-, 2′,4′-di-, 2′,5′-di-, 2′,6′-di- and 2′,4′,6′-tri-substituted acetophenones were generally more active than 3′4′-di-, 3′,5′-di-and 3′,4′,5′-tri-substituted ones. Although the hyphal growths of some fungi almost were inhibited completely by mono- and 2′,5′-di-substituted acetophenones, none of the fungi, with a few exceptions, were killed.
CONCLUSIONS:
2′,6′-Dihydroxyacetophenone and 2',6'-Dihydroxy-4'-methoxyacetophenone exhibited significant antifungal activities against all test fungi, whereas 2′-hydroxy-6′-methoxyacetophenone had limited activity. The ortho-hydroxyls to the acetyl in the molecules play an important role in antifungal activities, because methylation of the hydroxyls resulted in reduced activities. In contrast, the antifungal activities of 2′,4′-dihydroxyacetophenone and 2′,4′,6′-trihydroxyacetophenone were enhanced by the methylation of para-hydroxyl. The limited activity of 2′,4′,6′-trihydroxycetophenone suggested that one of the hydroxyls in the molecule was unfavorable for antifungal activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)