| In vitro: |
| Phytother Res. 2004 Feb;18(2):128-30. | | Antiplasmodial constituents of Cajanus cajan.[Pubmed: 15022164] |
METHODS AND RESULTS:
Bioactivity-guided fractionation of extracts of roots and leaves of Cajanus cajan afforded 8 compounds: betulinic acid, biochanin A, cajanol, genistein and 2'-Hydroxygenistein, longistylin A and C, and pinostrobin.
CONCLUSIONS:
The two stilbenes, longistylin A and C, and betulinic acid showed a moderately high in vitro activity against the chloroquine-sensitive Plasmodium falciparum strain 3D7. | | Bioorg Med Chem Lett. 2004 Feb 23;14(4):1011-4. | | Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica.[Pubmed: 15013012] | One new isoflavone, 5,7,4'-trihydroxy-2'-methoxyisoflavone (3) and seven, and four known compounds were isolated from the barks of Crotalaria pallida and the seeds of C. assamica, respectively.
METHODS AND RESULTS:
The known compounds, apigenin (1) and 2'-Hydroxygenistein (2), isolated from C. pallida, showed significant concentration-dependent inhibitory effects on the release of beta-glucuronidase and lysozyme from rat neutrophils in response to formyl-Met-Leu-Phe/cytochalasin B (fMLP/CB) with IC(50) values of 2.8+/-0.1 and 17.7+/-1.9, and 5.9+/-1.4 and 9.7+/-3.5 microM, respectively. The known compounds, daidzein (4) and 2'-hydroxydaidzein (6), isolated from C. pallida, inhibited of the release of lysozyme and beta-glucuronidase from rat neutrophils in response to fMLP/CB with IC(50) values of 26.3+/-5.5 and 13.7+/-2.6 microM, respectively. Compounds 1 and 4 also showed significant concentration-dependent inhibitory effects on superoxide anion generation in rat neutrophils stimulated with fMLP/CB with IC(50) values of 3.4+/-0.3 and 25.1+/-5.0 microM, respectively. Compounds 1 and 5, previously isolated from C. pallida, showed the inhibition of NO production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and LPS/interferon-gamma (IFN-gamma)-stimulated N9 microglial cells with IC(50) values of 10.7+/-0.1 and 13.9+/-1.1 microM, respectively.
CONCLUSIONS:
Flavonoids, suppressed chemical mediators in inflammatory cells, may have value in treatment and prevention of central and peripheral inflammatory diseases associated with excess production of chemical mediators. | | J Microbiol Biotechnol. 2009 Nov;19(11):1348-54. | | 2'-hydroxylation of genistein enhanced antioxidant and antiproliferative activities in mcf-7 human breast cancer cells.[Pubmed: 19996686] |
METHODS AND RESULTS:
Bioconversion of the isoflavonoid genistein to 2'-Hydroxygenistein (2'-HG) was performed using isoflavone 2'-hydroxylase (CYP81E1) heterologously expressed in yeast. A monohydroxylated product was analyzed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) and NMR spectrometry and was identified as 2'-HG. An initial bioconversion rate of 6% was increased up to 14% under optimized conditions. After recovery, the biological activity of 2'-HG was evaluated. Bioconverted 2'-HG showed higher antioxidant activity against 1,1- diphenyl-2-picryl hydrazine (DPPH) and 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radicals than did genistein. Furthermore, 2'-HG exhibited greater antiproliferative effects in MCF-7 human breast cancer cells than did genistein.
CONCLUSIONS:
These results suggest that 2'- hydroxylation of genistein enhanced its antioxidant activity and cell cytotoxicity in MCF-7 human breast cancer cells. |
|


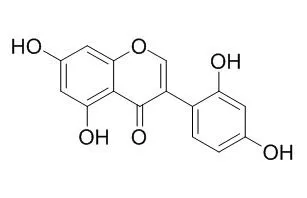



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)