| Structure Identification: |
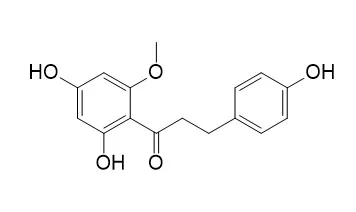
| J Agric Food Chem . 2003 May 21;51(11):3309-3312. | | Dihydrochalcones: evaluation as novel radical scavenging antioxidants[Pubmed: 12744659] | | Dihydrochalcones are a family of bicyclic flavonoids, defined by the presence of two benzene rings joined by a saturated three carbon bridge. In the present study, we systematically examined the antioxidant activities of dihydrochalcones against the stable free radical (1,1-diphenyl-2-picrylhydrazyl) and lipid peroxidation in the erythrocyte membrane. All dihydrochalcones exhibited higher antioxidant activities than the corresponding flavanones. The (1)H NMR analysis indicated that the active dihydrochalcone has a time-averaged conformation in which the aromatic A ring is orthogonal to the carbonyl group, while the inactive dihydrochalcone such as 2'-O-methyl-phloretin has a strongly hydrogen-bonded phenolic hydroxyl group, suggestive of a coplanar conformation. A hydroxyl group at the 2'-position of the dihydrochalcone A ring, newly formed by reduction of the flavanone C ring, is an essential pharmacophore for its radical scavenging potential. | | Phytochemistry . 2000 Nov;55(5):439-446. | | (Rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta, 4alpha-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii[Pubmed: 11140605] | | The aerial parts of Goniothalamus gardneri (Annonaceae) has yielded the known flavonoids 2'-hydroxy-4,4',6'-trimethoxychalcone (flavokawain A), 2',4'-dihydroxy-4,6'-dimethoxydihydrochalcone, 4,2',4'-trihydroxy-6'-methoxydihydrochalcone, 5,7,4'-trimethoxyflavanone (naringenin trimethyl ether) and 7-hydroxy-5,4'-dimethoxyflavanone (tsugafolin) together with three novel compounds, the dimer characterised as (rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta,4alpha-di-(4-methoxyphenyl)-cyclobutane, 2',4'-dihydroxy-4,6'-dimethoxychalcone and 2'-hydroxy-4,4',6'-trimethoxydihydrochalcone. The last two have previously been synthesised but appear to be new natural products. A similar study of the aerial parts of G. thwaitesii led only to the isolation of the known flavonoids myricetin 4'-O-methyl ether-3-O-alpha-L-rhamnopyranoside (mearnsitrin) and myricetin-3-O-methyl ether (annulatin), together with the triterpenes friedelinol, friedelin and betulinic acid. All compounds were identified by spectroscopic analysis and, for known compounds, by comparison with published data. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)