| Structure Identification: |
| Ind.eng.chem.res, 2008, 47(6):1774-1778. | | Obtaining 2Octanol, 2-Octanone, and Sebacic Acid from Castor Oil by Microwave-Induced Alkali Fusion.[Reference: WebLink] |
METHODS AND RESULTS:
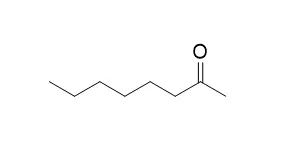
In this study, the effect of microwave irradiation on alkali fusion of castor oil was investigated using different NaOH/oil ratios (8:15, 12:15, and 14:15), reaction temperatures (473, 493, 503, 513, and 523 K), and reaction times (15, 20, 25, and 30 min) in order to obtain oleochemicals (2-octanol, 2-Octanone, and sebacic acid) in the presence of 1% catalyst (Pb3O4) by weight (w/w). The yields of 2-octanol (4.4−62.6%), 2-Octanone (1.6−37.4%), and sebacic acid (9.1−76.2%) were obtained according to reaction conditions.
Maximum yields of oleochemicals were obtained using methylated then presaponified castor oil (sodium ricinoleate), 14/15 NaOH/oil ratio at temperature 513 K and 20 min reaction time. 2-Octanol, 2-Octanone, and sebacic acid were determined by GC and GC/MS analysis, and purity of sebacic acid (98.7%) was further assessed by its acid value (444) and melting point (406.5 K).
CONCLUSIONS:
Reaction time compared to conventional heating was dramatically decreased by microwave heating system from 5 h reaction time to 20 min. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)