| Structure Identification: |
| Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(24):4329-34. | | Chemical constituents of Osmanthus fragrans fruits.[Pubmed: 24791540] |
METHODS AND RESULTS:
By Silica gel, Sephadex LH-20 and other materials for isolation and purification and by physicochemical methods and spectral analysis for structural identification, 23 compounds were isolated and identified from ethyl acetate portion of alcohol extract solution of Osmanthus fragrans fruits. Their structures were identified as nicotinamide (1), D-allitol (2), 5-hydroxymethyl-2-furancarboxaldehyde (3), acetyloleanolic acid (4), benzoic acid (5), ergosta-7,22-dien-3-one (6), beta-sitosterol (7), borreriagenin (8), cerevistero (9), c-veratroylglycol (10), methyl-2-O-beta-glucopyranosylbenzoate (11), 3', 7-dihydroxy-4'-methoxyisoflavon (12), umbelliferone (13), caffeic acid methyl ester (14), oleanolic acid (15), (-) -chicanine (16), dillapiol (17), 3beta,5alpha, 9alpha-trihydroxyergosta-7-22-dien-6-one (3,5,9-Trihydroxyergosta-7,22-dien-6-one,18), 2alpha-hydroxy-oleanolic acid (19), betulinic acid (20), betulin (21), 3, 3'-bisdemethylpinoresinol (22), and lupeol (23).
CONCLUSIONS:
All compounds were isolated from the osmanthus fruit for the first time. Except for compounds 4, 7, 15, 19, 23, the rest ones were isolated from the this plant for the first time. | | Bioorg Med Chem Lett. 2015 Nov 15;25(22):5078-82. | | Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells.[Pubmed: 26481911 ] |
METHODS AND RESULTS:
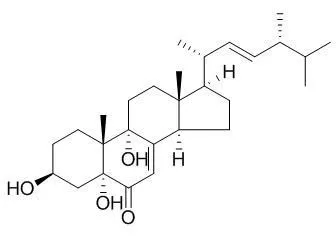
One new meroterpenoid, named hericenone K (11), along with 10 known compounds (1-10), ergosterol peroxide (1), cerevisterol (2), 3β,5α,9α-trihydroxy-ergosta-7,22-dien-6-one (3,5,9-Trihydroxyergosta-7,22-dien-6-one,3), inoterpene A (4), astradoric acid C (5), betulin (6), oleanolic acid (7), ursolic acid (8), hemisceramide (9), and 3,4-dihydro-5-methoxy-2-methyl-2-(4'-methyl-2'-oxo-3'-pentenyl)-9(7H)-oxo-2H-furo[3,4-h]benzopyran (10), was isolated from the fruiting bodies of the mushroom Hericium erinaceus.
Their structures were characterized on the basis of spectroscopic methods, as well as through comparison with previously reported data. Compounds 3-6, 8, and 9 were isolated from Hericium species for the first time.
CONCLUSIONS:
Compounds 10 and 11 was suggested to be racemic by the CD spectrum data and specific rotations, which ware resolved by chiral HPLC into respective enantiomers. Compounds 1-3, (±)-10, (-)-10 and (+)-10 in the presence of NGF (20 ng/mL) exerted a significant increase in neurite-bearing cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)