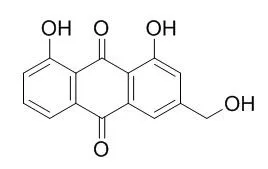
| Description: |
Aloeemodin is an interferon-inducing agent with IC50 of about 1 μg/mL for JEV and of about 0.33 μg/mL for EV71. Aloeemodin has antitumor, neuroprotective, and anti-fibrosis effects, it inhibited β-amyloid aggregation, downregulated the expression of Smad2 mRNA and TGF-β1,TIMP1,and type Ⅰ and Ⅲ collagen proteins,and upregulated the expression of Smad7 mRNA. |
| Targets: |
Calcium Channel | ROS | Beta Amyloid | TGF-β/Smad | MMP(e.g.TIMP) | FAK | VEGFR |
| In vitro: |
| Mutat Res. 1996 Mar 1;367(3):123-33. | | Genotoxicity of aloeemodin in vitro and in vivo.[Pubmed: 8600368] | The present in vitro and in vivo experiments were undertaken to clarify the genotoxic potential of the hydroxyanthrachinone Aloeemodin which can be found in different plant derived products for therapy of constipation.
METHODS AND RESULTS:
The results demonstrate that Aloeemodin is able to induce mutagenic effects in vitro. Positive results were obtained in the chromosomal aberration assay with CHO cells, as well as in the Salmonella reverse mutation assay (frameshift mutations in strains TA 1537, TA 1538 and TA 98). No mutagenic potential of Aloeemodin, however, was observed in the gene mutation assay with mammalian cells in vitro (HPRT assay in V79 cells). Each assay was performed in the presence and absence of an extrinsic metabolic activation system (S9-mix). In in vivo studies (micronucleus assay in bone marrow cells of NMRI mice; chromosome aberration assay in bone marrow cells of Wistar rats; mouse spot text [DBA/2JxNMRI]) no indication of a mutagenic activity of Aloeemodin was found. Information about a possible reaction of Aloeemodin with DNA was derived from an in vivo UDS assay. Hepatocytes of Aloeemodin-treated male Wistar rats did not show DNA damage via repair synthesis.
CONCLUSIONS:
All these data suggest that Aloeemodin is able to interact with DNA under certain in vitro conditions. However, in vivo the results that were negative did not indicate a genotoxic potential. Therefore, it may be assumed that a genotoxic risk for man might be unlikely. | | Curr Alzheimer Res. 2015;12(5):424-33. | | Inhibition of β-Amyloid Aggregation by Albiflorin, Aloeemodin and Neohesperidin and their Neuroprotective Effect on Primary Hippocampal Cells Against β-Amyloid Induced Toxicity.[Pubmed: 25938872] | Being one of the hallmarks of Alzheimer's disease, β-amyloid (Aβ) aggregates induce complicated neurotoxicity. Evidences show that the underlying mechanism of neurotoxicity involves a glutamate receptor subtype, N-methyl-D-aspartate (NMDA) receptor, an increase in intracellular calcium(II) ion loading as well as an elevation in oxidation stress.
METHODS AND RESULTS:
In this work, among the 35 chemical components of Chinese herbal medicines (CHMs) being screened for inhibitors of Aβ aggregation, four of them, namely albiflorin, Aloeemodin, neohesperidin and physcion, were found for the first time to exhibit a potent inhibitory effect on Aβ(1-40) and Aβ(1-42) aggregation. Their neuroprotective capability on primary hippocampal neuronal cells was also investigated by MTT assay, ROS assay and intracellular calcium(II) ion concentration measurement.
CONCLUSIONS:
It was interesting to find that physcion was rather toxic to neuronal cells while albiflorin, Aloeemodin and neohesperidin reduced the toxicity and ROS induced by both monomeric and oligomeric Aβ species. In addition, albiflorin was particularly powerful in maintaining the intracellular Ca(2+) concentration. |
|
| In vivo: |
| World Chinese Journal of Digestology, 2009, 17(27):2778-83. | | Effects of combined use of aloeemodin and praziquantel on the transforming growth factor-β/Smad pathway in mice with schistosomiasis-induced liver fibrosis.[Reference: WebLink] | To investigate the effects of combined use of Aloeemodin and praziquantel on the transforming growth factor-β (TGF-β)/Smad pathway in mice with schistosomiasis-induced liver fibrosis.
METHODS AND RESULTS:
Eighty mice were randomly divided into four groups: normal control group, infection group, praziquantel treatment group, and praziquantel and Aloeemodin treatment group. Mice in the infection group and the two treatment groups were infected with 25 Schistosoma japonicum cercariae. Eight weeks after infection, mice in the praziquantel treatment group were treated with praziquantel at a dose of 500 mg/(kg·d) for two days, while those in the praziquantel and Aloeemodin treatment group were treated with praziquantel at the same dose for the same duration followed by treatment with Aloeemodin at a dose of 0.3 mg/(kg·d) for 8 weeks. At week 16, all mice were sacrificed to take liver tissue samples. Hematoxylin and eosin staining was performed to observe changes in hepatic histopathology. Reverse transcription-polymerase chain reaction (RT-PCR) was used to detect the expression of Smad2 and Smad7 mRNAs in the liver. Immunohistochemical staining was performed to detect the expression of TGF-β1, TIMP-1, and type I and III collagen in liver tissue. Aloeemodin treatment relieved the degree of hepatic fibrosis. The expression levels of Smad2 mRNA and TGF-β1, tissue inhibitor of metalloproteinases-1 (TIMP1), and type I and III collagen proteins in liver tissue were significantly lower in the praziquantel and Aloeemodin treatment group than in the infection group and the praziquantel treatment group (q = 6.20 and 4.38, 6.22 and 4.41, 6.30 and 4.52, 6.25 and 4.44. and 6.29 and 4.48, respectively; all P < 0.01 or 0.05). In contrast, the expression level of Smad7 mRNA was significantly higher in the praziquantel and Aloeemodin treatment group than in the infection group and the praziquantel treatment group (q = 6.32 and 4.62, respectively; P < 0.01 and 0.05, respectively).
CONCLUSIONS:
Aloeemodin exerts anti-fibrotic effects perhaps through downregulation of the expression of Smad2 mRNA and TGF-β1, TIMP1, and type I and III collagen proteins, and upregulation of the expression of Smad7 mRNA. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)