| Description: |
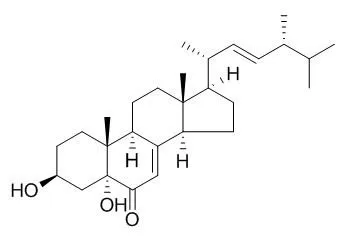
3β,5α-Dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one exhibits strong or moderate cytotoxic activities against MCF-7, A549, Hela and KB cell lines with IC50 values 4.98 (MCF-7), 1.95 (A549), 0.68(Hela), and 1.50 uM (KB), respectively. |
| In vitro: |
| Marine Drugs,2013,11(7):2616-24. | | Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea[Reference: WebLink] |
METHODS AND RESULTS:
The mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) was cultivated in potato dextrose liquid medium, and one rare thiophene compound (1), together with anhydrojavanicin (2), 8-O-methylbostrycoidin (3), 8-O-methyljavanicin (4), botryosphaerone D (5), 6-ethyl-5-hydroxy-3,7-dimethoxynaphthoquinone (6), 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (3,5-Dihydroxyergosta-7,22-dien-6-one, 7), 3β,5α,14α-trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (8), NGA0187 (9) and beauvericin (10), were isolated. Their structures were elucidated by analysis of spectroscopic data. This is the first report of a natural origin for compound 6. Moreover, compounds 3, 4, 5, 7, 8 and 10 were obtained from marine microorganism for the first time.
CONCLUSIONS:
In the bioactive assays in vitro, compounds 2, 3, 9 and 10 displayed remarkable inhibiting actions against α-acetylcholinesterase (AChE) with IC50 values 2.01, 6.71, 1.89, and 3.09 μM, respectively. Furthermore, in the cytotoxicity assays, compounds 7 and 10 exhibited strong or moderate cytotoxic activities against MCF-7, A549, Hela and KB cell lines with IC50 values 4.98 and 2.02 (MCF-7), 1.95 and 0.82 (A549), 0.68 and 1.14 (Hela), and 1.50 and 1.10 μM (KB), respectively; compound 8 had weak inhibitory activities against these tumor cell lines; compounds 1, 2, 3, 4, 5, 6 and 9 exhibited no inhibitory activities against them. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)