| Structure Identification: |
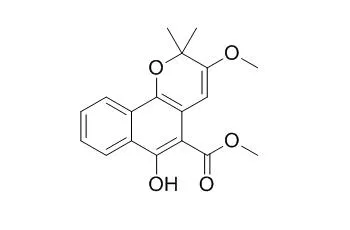
| J Org Chem. 2010 Apr 2;75(7):2274-80. | | Synthesis of the natural products 3-hydroxymollugin and 3-methoxymollugin.[Pubmed: 20201485 ] | 3-Hydroxymollugin 2 and 3-Methoxymollugin 3 are cytotoxic compounds isolated as minor compounds from Pentas longiflora and Rubia cordifolia.
METHODS AND RESULTS:
Syntheses of 3-hydroxymollugin 2 and 3-Methoxymollugin 3 were developed starting from easily available 3-bromomollugin 6. Surprisingly, it was found that the reaction of 3-bromomollugin 6 with sodium methoxide in methanol resulted in the formation of 3-Methoxymollugin 3 and the ring-contracted methyl isopropenylfuromollugin 7. A mechanism for this ring contraction is proposed on the basis of a pericyclic retro oxa-6pi ring-opening reaction. A second synthesis of 3-hydroxymollugin 2 was based on epoxidation of methyl 3-(3-methylbut-2-enyl)-1,4-naphthoquinone-2-carboxylate 17 and subsequent reduction of the quinone moiety, ring transformation, and DDQ oxidation. The latter oxidation process results in 3-hydroxymollugin 2 along with the rearranged furomollugin 4, which is a ring-contracted analogue of the natural product mollugin 1. | | J Nat Prod. 2002 Sep;65(9):1377-9. | | New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora.[Pubmed: 12350172] |
METHODS AND RESULTS:
Several quinone type compounds were isolated from the hexane, dichloromethane, and ethyl acetate extracts of the roots of Pentas longiflora. The hexane extract afforded two new compounds, [(3alpha,3'alpha,4beta,4'beta)-3,3']-dimethoxy-cis-[4,4'-bis(3,4,5,10-tetrahydro-1H-naphtho[2,3-c]pyran)]-5,5',10,10'-tetraone (1) and cis-3,4-dihydroxy-3,4-dihydromollugin (2), together with six known compounds, namely, pentalongin, mollugin, trans-3,4-dihydroxy-3,4-dihydromollugin, methyl-2,3-epoxy-3-prenyl-1,4-naphthoquinone-2-carboxylate, tectoquinone, and 3-hydroxymollugin.
CONCLUSIONS:
From the dichloromethane extract were isolated the three known compounds 3-Methoxymollugin, methyl-3-prenyl-1,4-naphthoquinone-2-carboxylate, and scopoletin, while the ethyl acetate extract afforded the known 2-methoxy-3-methylanthraquinone. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)