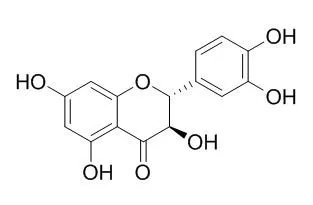
| Description: |
Taxifolin exhibits important anti-tyrosinase activity, it also exhibits significant inhibitory activity against collagenase with an IC50 value of 193.3 μM.Taxifolin has anti-oxidant, anti-melanogenic, chemopreventive, anti-inflammatory, and cardioprotective effects. Taxifolin promotes osteoblast differentiation in MC3T3-E1 cells and also inhibit osteoclastogenesis in RAW264.7 cells, it also can enhance andrographolide-induced mitotic arrest and apoptosis in human prostate cancer cells via spindle assembly checkpoint activation.
|
| In vitro: |
| Cancer Prev Res (Phila). 2012 Sep;5(9):1103-14. | | Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K.[Pubmed: 22805054 ] | Skin cancer is one of the most commonly diagnosed cancers in the United States. Taxifolin reportedly exerts multiple biologic effects, but the molecular mechanisms and direct target(s) of Taxifolin in skin cancer chemoprevention are still unknown.
METHODS AND RESULTS:
In silico computer screening and kinase profiling results suggest that the EGF receptor (EGFR), phosphoinositide 3-kinase (PI3K), and Src are potential targets for Taxifolin. Pull-down assay results showed that EGFR, PI3K, and Src directly interacted with Taxifolin in vitro, whereas Taxifolin bound to EGFR and PI3K, but not to Src in cells. ATP competition and in vitro kinase assay data revealed that Taxifolin interacted with EGFR and PI3K at the ATP-binding pocket and inhibited their kinase activities. Western blot analysis showed that Taxifolin suppressed UVB-induced phosphorylation of EGFR and Akt, and subsequently suppressed their signaling pathways in JB6 P+ mouse skin epidermal cells. Expression levels and promoter activity of COX-2 and prostaglandin E(2) (PGE(2)) generation induced by UVB were also attenuated by Taxifolin. The effect of Taxifolin on UVB-induced signaling pathways and PGE(2) generation was reduced in EGFR knockout murine embryonic fibroblasts (MEF) compared with EGFR wild-type MEFs. Taxifolin also inhibited EGF-induced cell transformation. Importantly, topical treatment of Taxifolin to the dorsal skin significantly suppressed tumor incidence, volume, and multiplicity in a solar UV (SUV)-induced skin carcinogenesis mouse model. Further analysis showed that the Taxifolin-treated group had a substantial reduction in SUV-induced phosphorylation of EGFR and Akt in mouse skin.
CONCLUSIONS:
These results suggest that Taxifolin exerts chemopreventive activity against UV-induced skin carcinogenesis by targeting EGFR and PI3K. | | PLoS One. 2013;8(1):e54577. | | Taxifolin enhances andrographolide-induced mitotic arrest and apoptosis in human prostate cancer cells via spindle assembly checkpoint activation.[Pubmed: 23382917] | Andrographolide (Andro) suppresses proliferation and triggers apoptosis in many types of cancer cells. Taxifolin (Taxi) has been proposed to prevent cancer development similar to other dietary flavonoids.
METHODS AND RESULTS:
In the present study, the cytotoxic and apoptotic effects of the addition of Andro alone and Andro and Taxi together on human prostate carcinoma DU145 cells were assessed. Andro inhibited prostate cancer cell proliferation by mitotic arrest and activation of the intrinsic apoptotic pathway. Although the effect of Taxi alone on DU145 cell proliferation was not significant, the combined use of Taxi with Andro significantly potentiated the anti-proliferative effect of increased mitotic arrest and apoptosis by enhancing the cleavage of poly(ADP-ribose) polymerase, and caspases-7 and -9. Andro together with Taxi enhanced microtubule polymerization in vitro, and they induced the formation of twisted and elongated spindles in the cancer cells, thus leading to mitotic arrest. In addition, we showed that depletion of MAD2, a component in the spindle assembly checkpoint (SAC), alleviated the mitotic block induced by the two compounds, suggesting that they trigger mitotic arrest by SAC activation.
CONCLUSIONS:
This study suggests that the anti-cancer activity of Andro can be significantly enhanced in combination with Taxi by disrupting microtubule dynamics and activating the SAC. | | Molecules . 2016 Nov 21;21(11):1586. | | Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties[Pubmed: 27879645] | | Abstract
Common spruce (Picea abies L.) is a fast-growing coniferous tree, widely used in several countries for the production of sawn wood, timber and pulp. During this industrial exploitation, large quantities of barks are generated as waste materials. The aim of this study was the bio-guided investigation and the effective recovery of methanol-soluble metabolites of common spruce bark for the development of new dermo-cosmetic agents. The active methanol extract was initially fractionated by Centrifugal Partition Chromatography (CPC) using a triphasic solvent system in a step-gradient elution mode. All resulting fractions were evaluated for their antibacterial activity, antioxidant activity and their capability to inhibit tyrosinase, elastase and collagenase activity. In parallel, the chemical composition of each fraction was established by combining a 13C-NMR dereplication approach and 2D-NMR analyses. As a result, fourteen secondary metabolites corresponding to stilbene, flavonoid and phenolic acid derivatives were directly identified in the CPC fractions. A high amount (0.93 g) of E-astringin was recovered from 3 g of crude extract in a single 125 min run. E-Astringin significantly induced the tyrosinase activity while E-piceid, Taxifolin, and Taxifolin-3'-O-glucopyranoside exhibited significant anti-tyrosinase activity. The above compounds showed important anti-collagenase and antimicrobial activities, thus providing new perspectives for potential applications as cosmetic ingredients.
Keywords: 13C-NMR dereplication; Centrifugal Partition Chromatography; E-astringin; dermo-cosmetic agents; Taxifolin; tyrosinase, elastase and collagenase activity. | | Front Pharmacol . 2021 Jan 14;11:608511. | | Dissecting Efficacy and Metabolic Characteristic Mechanism of Taxifolin on Renal Fibrosis by Multivariate Approach and Ultra-Performance Liquid Chromatography Coupled With Mass Spectrometry-Based Metabolomics Strategy[Pubmed: 33519473] | | Abstract
Taxifolin (TFN) is an important natural compound with antifibrotic activity; however, its pharmacological mechanism is not clear. In this study, our aim is to gain insight into the effects of TFN and its potential mechanisms in unilateral ureteral obstruction (UUO) animal model using metabolomics approach to identify the metabolic biomarkers and perturbed pathways. Serum metabolomics analysis by UPLC-Q-TOF/MS was carried out to discover the changes in the metabolic profile. It showed that TFN has a significant protective effect on UUO-induced renal fibrosis and a total of 32 potential biomarkers were identified and related to RF progression. Of note, 27 biomarkers were regulated by TFN treatment, which participate in eight metabolic pathways, including phenylalanine, tyrosine and tryptophan biosynthesis, and phenylalanine metabolism. It also showed that metabolomics was a promising strategy to better dissect metabolic characteristics and pharmacological mechanisms of natural compounds by multivariate approach and ultra-performance liquid chromatography coupled with mass spectrometry.
Keywords: UPLC-Q-TOF/MS; biomarker; metabolic pathway; metabolomics; natural product; renal fibrosis. |
|
| In vivo: |
| Food Chem Toxicol. 2014 Jan;63:221-32. | | Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis.[Pubmed: 24269735] | Diabetic cardiomyopathy has been increasingly recognized as an important cause of heart failure in diabetic patients. Excessive oxidative stress has been suggested to play a critical role in the development of diabetic cardiomyopathy.
METHODS AND RESULTS:
The objective of this study was to investigate the potential protective effects and mechanisms of Taxifolin on cardiac function of streptozotocin-induced diabetic mice and on hyperglycemia-induced apoptosis of H9c2 cardiac myoblasts. In vivo study revealed that Taxifolin improved diastolic dysfunction, ameliorated myocardium structure abnormality, inhibited myocyte apoptosis and enhanced endogenous antioxidant enzymes activities. Interestingly, Taxifolin reduced angiotensin II level in myocardium, inhibited NADPH oxidase activity, and increased JAK/STAT3 activation. In vitro investigation demonstrated that Taxifolin inhibited 33 mM glucoseinduced H9c2 cells apoptosis by decreasing intracellular ROS level. It also inhibited caspase-3 and caspase-9 activation, restored mitochondrial membrane potential, and regulated the expression of proteins related to the intrinsic pathway of apoptosis, thus inhibiting the release of cytochrome c from mitochondria into the cytoplasm.
CONCLUSIONS:
In conclusion, Taxifolin exerted cardioprotective effects against diabetic cardiomyopathy by inhibiting oxidative stress and cardiac myocyte apoptosis and might be a potential agent in the treatment of diabetic cardiomyopathy. | | Phytother Res. 2008 Sep;22(9):1200-7. | | Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels.[Pubmed: 18729255 ] | | Flavonoids are a group of polyphenolic compounds widely distributed in plants. Their potent bio-activities and relatively low toxicity have rendered them useful ingredients in functional cosmetics. The purpose of the present study was to examine their potential effects on cellular melanogenesis. When tested in murine melanoma B16F10 cells activated by alpha-melanocyte stimulating hormone (alpha-MSH), Taxifolin and luteolin inhibited the cellular melanogenesis as effectively as arbutin, one of the most widely used hypopigmenting agents in cosmetics. As opposed to their antimelanogenic effects, Taxifolin and luteolin rather increased the tyrosinase protein levels in the absence and presence of alpha-MSH. However, these flavonoids effectively inhibited tyrosinase-catalysed oxidation of l-dihydroxyphenylalanine in cell-free extracts and in living cells. Furthermore, they attenuated cell pigmentation induced by expression of exogenous human tyrosinase. Therefore, the antimelanogenic effects of Taxifolin and luteolin are attributed to their inhibitory effects on tyrosinase enzymatic activity, despite their effects on increasing tyrosinase protein levels. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)