| Structure Identification: |
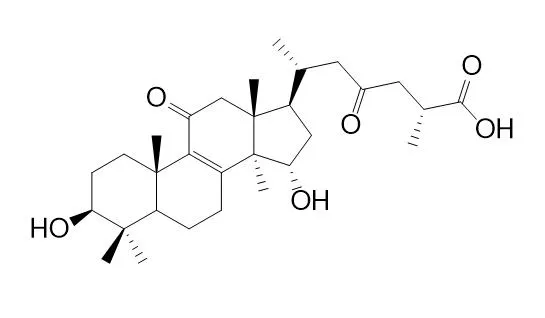
| Zhongguo Zhong Yao Za Zhi . 2016 Mar;41(6):1075-1080. | | [Triterpenoids from Ganoderma theaecolum][Pubmed: 28875673] | | Fifteenlanostane triterpenoids were isolated from the ethanol extract of Ganoderma theaecolum by means of preparative HPLC,column chromatography over silica gel,ODS and were identified as lucidone C(1),lucidone D(2),7-oxo-ganoderic acid Z2(3),7-oxo-ganoderic acid Z(4),ganoderenicacid H(5),ganoderenic acid B(6),3β,7β-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid(7),3β,7β-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid methyl ester(8),Ganolucidic acid B(9),ganolucidate F(10),methyl ganoderate C2(11),ganoderic acid ζ(12),ganoderic acid AP3(13),methyl ganoderate B(14),and ganoderol B(15). Compounds 1-15 were isolated from this specie for the first time. | | Planta Med . 2012 Mar;78(4):368-376. | | Isolation and bioactivity evaluation of terpenoids from the medicinal fungus Ganoderma sinense[Pubmed: 22161763] | | A new pentanorlanostane, ganosineniol A (1), eight new lanostane triterpenoids, ganosinoside A (2), ganoderic acid Jc (3), ganoderic acid Jd (4), ganodermatetraol (5), ganolucidic acid γa (6), ganolucidate F (7), ganoderiol J ( 8), and methyl lucidenate Ha ( 9), and a new sesquiterpenoid, ganosinensine (10), together with eleven known triterpenoids (11- 21), were isolated from the fruiting bodies of the fungus Ganoderma sinense. Chemical structures were determined based on spectroscopic evidence, including 1D, 2D NMR, and mass spectral data. Furthermore, all isolates were tested for cytotoxic activity and induction ability of hPXR-mediated CYP3A4 expression. Among them, ganoderic acid Jc (3) displayed selective inhibitory activity against HL-60 cells (IC₅₀ = 8.30 μM), and ganoderiol E (11) exhibited selective cytotoxic activity against MCF-7 cells (IC₅₀ = 6.35 μM). Meanwhile, compounds 5, 7, and ganolucidic acids B and C (19, 20) showed induction ability of hPXR-mediated CYP3A4 expression. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)