| Description: |
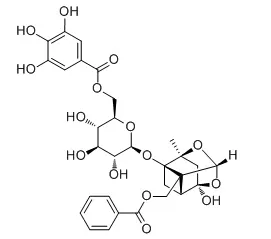
6'-O-Galloylpaeoniflorin (GPF) may be developed as a cytoprotector against ROS-mediated oxidative stress, GPF demonstrates a significant scavenging capacity against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, H2O2-generated intracellular reactive oxygen species (ROS), the superoxide anion radical (O2 -), and the hydroxyl radical (•OH); GPF also can safeguarde HaCaT keratinocytes against H2O2-provoked apoptotic cell death and attenuated oxidative macromolecular damage to DNA, lipids, and proteins; GPF exerts its cytoprotective actions in keratinocytes at least in part by decreasing the number of DNA strand breaks, the levels of 8-isoprostane (a stable end-product of lipid peroxidation), and the formation of carbonylated protein species. GPF shows strong androgen receptor (AR) binding activity. |
| In vitro: |
| Biomol. Ther., 2013, 21(5):349-57. | | 6'-o-galloylpaeoniflorin protects human keratinocytes against oxidative stress-induced cell damage.[Pubmed: 24244822 ] | 6'-O-Galloyl paeoniflorin (GPF) is a galloylated derivate of paeoniflorin and a key chemical constituent of the peony root, a perennial flowering plant that is widely used as an herbal medicine in East Asia.
METHODS AND RESULTS:
This study is the first investigation of the cytoprotective effects of GPF against hydrogen peroxide (H2O2)-induced cell injury and death in human HaCaT keratinocytes. GPF demonstrated a significant scavenging capacity against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, H2O2-generated intracellular reactive oxygen species (ROS), the superoxide anion radical (O2 (-)), and the hydroxyl radical (•OH). GPF also safeguarded HaCaT keratinocytes against H2O2-provoked apoptotic cell death and attenuated oxidative macromolecular damage to DNA, lipids, and proteins. The compound exerted its cytoprotective actions in keratinocytes at least in part by decreasing the number of DNA strand breaks, the levels of 8-isoprostane (a stable end-product of lipid peroxidation), and the formation of carbonylated protein species.
CONCLUSIONS:
Taken together, these results indicate that GPF may be developed as a cytoprotector against ROS-mediated oxidative stress. | | Chem. Pharm. Bull., 2009, 57(9):971-4. | | Androgen modulators from the roots of Paeonia lactiflora (paeoniae radix) grown and processed in nara prefecture, Japan.[Pubmed: 19721258] | The monoterpene glycoside, 3'-O-galloylpaeoniflorin (1), and four known compounds, 6'-O-galloylalbiflorin (2), pentagalloylglucose (3), 6'-O-benzoylpaeoniflorin (4) and 6'-O-Galloyl paeoniflorin (5), were isolated from the roots of Paeonia lactiflora that had been grown and processed in Nara prefecture, Japan, as androgen modulators.
METHODS AND RESULTS:
Their structures were elucidated based on spectroscopic analysis. Compounds 2 and 3 showed strong androgen receptor (AR) binding activity (IC(50) values 33.7 and 4.1 microg/ml, respectively), 1, 4 and 5 showed weak activity (20, 31 and 12% at 120 microg/ml, respectively). However, paeoniflorin (6) and albiflorin (7), the structures of which are related to 1, 2, 4 and 5, showed no activity.
CONCLUSIONS:
These results suggested that both the structure of albiflorin and the galloyl moiety are important for 2 to show strong AR binding activity. Furthermore, compounds 1-5 inhibited growth of an androgen-dependent LNCaP-FGC (prostate cancer cell line), and were indicated to be AR antagonists. Compounds 2 and 3 might be candidates as safe, natural anti-androgens. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)