| J Sep Sci. 2005 Nov;28(17):2288-92. |
| Simultaneous densitometric determination of artemisinin, artemisinic acid and arteannuin-B in Artemisia annua using reversed-phase thin layer chromatography.[Pubmed: 16342793] |
METHODS AND RESULTS:
A rapid and simple RP-TLC method for simultaneous quantification of pharmacologically important sesquiterpene artemisinin (AM) together with its precursors Arteannuin B (AB) and artemisinic acid (AA) in the inflorescence part of Artemisia annua plant has been developed. The RP-TLC of sesquiterpenes was performed on RP-18 F254 S thin-layer chromatographic plates by developing in mobile phase, containing 0.2% TFA in water/ACN (35:65, v/v). The densitometric determination of AM, AB and AA was carried out after derivatization with anisaldehyde reagent at 426 nm in absorption-reflectance mode. |
| J Nat Prod. 1993 Sep;56(9):1559-66. |
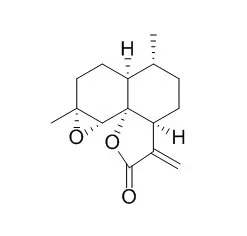
| Bioconversion of arteannuin B to artemisinin.[Pubmed: 8254350] |
| Arteannuin B, which co-occurs with artemisinin, the potent antimalarial principle of the Chinese medicinal herb Artemisia annua (Asteraceae), has been converted to the latter using crude and semi-purified cell-free extracts of the leaf homogenates of the plant. Detection procedures to quantitate this bioconversion, including one that is novel which uses gcms, are detailed. |
| 8. IUPAC symposium on organometallic chemistry directed towards organic synthesis, Santa Barbara, CA (United States), 6-10 Aug 1995. |
| Samarium(II) induced asymmetric reductive cyclizations: The total synthesis of (-)-C{sub 10} desmethyl arteannuin B[Reference: WebLink] |
Arteannuin B is a member of the qinghaosu family, a novel class of sesquiterpenes that exhibit powerful antimalarial activity even against chloroquinine resistant strains. It is readily convertible to qinghaosu in several high yielding steps and has potential antitumor activity. Several approaches to the construction of the cis-decalin backbone have involved the use of electrochemical and metal promoted reductions as well as alkylations.
METHODS AND RESULTS:
We report a short convenient total synthesis of (-)-C{sub 10} desmethyl Arteannuin B utilizing an asymmetric reductive cyclization with samarium (II) iodide which selectively forms the cis-decalin ring structure while setting the trans relationship between the subunits of the {gamma}-hydroxy ester. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)