| Animal Research: |
| Antimicrob Agents Chemother. 2014 Oct;58(10):5658-65. | | Enhanced antimalarial activity by a novel artemether-lumefantrine lipid emulsion for parenteral administration.[Pubmed: 24982079] | Artemether and lumefantrine (also known as benflumetol) are difficult to formulate for parenteral administration because of their low aqueous solubility. Cremophor EL as an emulsion excipient has been shown to cause serious side effects.
METHODS AND RESULTS:
This study reports a method of preparation and the therapeutic efficacies of novel lipid emulsion (LE) delivery systems with Artemether, lumefantrine, or Artemether in combination with lumefantrine, for parenteral administration. Their physical and chemical stabilities were also evaluated. Furthermore, the in vivo antimalarial activities of the lipid emulsions developed were tested in Plasmodium berghei-infected mice. Artemether, lumefantrine, or Artemether in combination with lumefantrine was encapsulated in an oil phase, and the in vivo performance was assessed by comparison with artesunate for injection. It was found that the lumefantrine lipid emulsion (LUM-LE) and Artemether-lumefantrine lipid emulsion (ARM-LUM-LE-3) (1:6) began to decrease the parasitemia levels after only 3 days, and the parasitemia inhibition was 90% at doses of 0.32 and 0.27 mg/kg, respectively, with immediate antimalarial effects greater than those of the positive-control group and constant antimalarial effects over 30 days.
CONCLUSIONS:
LUM-LE and ARM-LUM-LE-3 demonstrated the best performance in terms of chemical and physical stabilities and antiplasmodial efficacy, with a mean particle size of 150 nm, and they have many favorable properties for parenteral administration, such as biocompatibility, physical stability, and ease of preparation. | | Am J Trop Med Hyg. 1994 Sep;51(3):251-9. | | Fatal neurotoxicity of arteether and artemether.[Pubmed: 7943542] | | Artemisinin (qinghaosu) and several derivatives have been developed and are in use as antimalarial drugs but scant information is available regarding animal or human toxicity. Following a eight-day, multiple-dose, pharmacokinetic study of arteether (AE) (10 mg/kg/day [n = 6] and 20 mg/kg/day [n = 6]) in dogs, all high-dose animals displayed a progressive syndrome of clinical neurologic defects with progressive cardiorespiratory collapse and death in five of six animals. Neurologic findings included gait disturbances, loss of spinal and pain response reflexes, and prominent loss of brain stem and eye reflexes. Animals had prolongation of QT interval corrected for rate (QTc) on electrocardiograms (ECGs) with bizarre ST-T segment changes. Prominent neuropathic lesions were noted to be primarily limited to the pons and medulla. Similar lesions with dose-related severity were noted in eight other dogs studied in a second study with intramuscular (IM) administration of AE in sesame oil during a 28-day, dose-ranging study using 5, 10, 15, and 20 mg/kg/day. Injury, graded by a pathologist blinded to the dose group, showed a dose-related, region-specific injury in all animals that was most pronounced in the pons. Further studies in Sprague-Dawley rats using IM administration of AE and Artemether (AM) at a dose of 12.5-50 mg/kg/day for 28 days confirmed the onset of a clinical neurologic syndrome with dose-related changes in body weight, activity, and seizure-like activity, stereotypic movement disorders, and ECG changes |
|


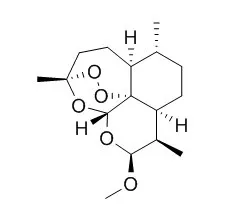



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)