| Structure Identification: |
| Chem Pharm Bull (Tokyo). 2010 Mar;58(3):438-41. | | Structural revisions of blumenol C glucoside and byzantionoside B.[Pubmed: 20190461 ] |
METHODS AND RESULTS:
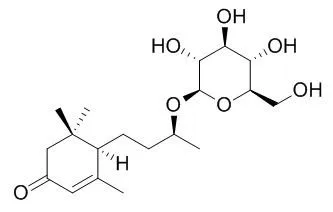
The absolute stereochemistry of Blumenol C glucoside and byzantionoside B was revised here as (6R,9S)- and (6R,9R)-9-hydroxymegastigman-4-en-3-one 9-O-beta-D-glucopyranosides, respectively, by modified Mosher's method. The empirical rules of (13)C-NMR chemical shift to determine the absolute stereochemistry of C-9 of 9-hydroxymegastigmane 9-O-beta-D-glucopyranoside were also discussed. | | Zhongguo Zhong Yao Za Zhi. 2012 May;37(10):1417-21. | | [Chemical constituents from the seed coat of Juglans regia].[Pubmed: 22860453] |
METHODS AND RESULTS:
Fifteen compounds were isolated from the seed coat of Juglans regia by silica gel, MCI gel and Sephadex LH-20 gel column chromatography, as well as high preparative performance liquid chromatography.
CONCLUSIONS:
Their structures were identified as salidroside (1), (6S, 9S)-roseoside (2), (6S, 9R)-roseoside (3), Blumenol C glucoside (4), byzantionoside B (5), 5-hydroxy-2-methoxy-1, 4-naphthoquinone (6), gallic acid (7), glycerol 1-(9Z-octadecenoate)-2-(9Z, 12Z-octadecadienoate)-3-(9Z, 12Z, 15Z-octadecatrienoate) (8), glycerol 1, 2, 3-tri-(9Z, 12Z-octadecadienoate) (9), glycerol 1, 2, 3-tri-(9Z, 12Z, 15Z-octadecatrienoate) (10), glycerol 1-hexadecanoate-2, 3-di-(9Z, 12Z-octadecadienoate) (11) on the basis of EI-MS, FAB-MS and NMR spectra. Moreover, 35 volatile compounds were identified by GC-MS. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)