Glucosinolates represent a large group of plant natural products long known for diverse and fascinating physiological functions and activities. Despite the relevance and huge interest on the roles of indole glucosinolates in plant defense, little is known about their direct interaction with microbial plant pathogens.
METHODS AND RESULTS:
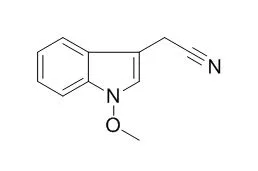
Toward this end, the metabolism of indolyl glucosinolates, their corresponding desulfo-derivatives, and derived metabolites, by three fungal species pathogenic on crucifers was investigated. While glucobrassicin, 1-methoxyglucobrassicin, 4-methoxyglucobrassicin were not metabolized by the pathogenic fungi Alternaria brassicicola, Rhizoctonia solani and Sclerotinia sclerotiorum, the corresponding desulfo-derivatives were metabolized to indolyl-3-acetonitrile, Caulilexin C (1-methoxyindolyl-3-acetonitrile) and arvelexin (4-methoxyindolyl-3-acetonitrile) by R. solani and S. sclerotiorum, but not by A. brassicicola. That is, desulfo-glucosinolates were metabolized by two non-host-selective pathogens, but not by a host-selective. Indolyl-3-acetonitrile, Caulilexin C and arvelexin were metabolized to the corresponding indole-3-carboxylic acids. Indolyl-3-acetonitriles displayed higher inhibitory activity than indole desulfo-glucosinolates. Indolyl-3-methanol displayed antifungal activity and was metabolized by A. brassicicola and R. solani to the less antifungal compounds indole-3-carboxaldehyde and indole-3-carboxylic acid.
CONCLUSIONS:
Diindolyl-3-methane was strongly antifungal and stable in fungal cultures, but ascorbigen was not stable in solution and displayed low antifungal activity; neither compound appeared to be metabolized by any of the three fungal species.
The cell-free extracts of mycelia of A. brassicicola displayed low myrosinase activity using glucobrassicin as substrate, but myrosinase activity was not detectable in mycelia of either R. solani or S. sclerotiorum. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)