| In vitro: |
| J Nat Prod. 2013 Jan 25;76(1):79-84. | | Meroterpenoid pigments from the basidiomycete Albatrellus ovinus.[Pubmed: 23305465] |
METHODS AND RESULTS:
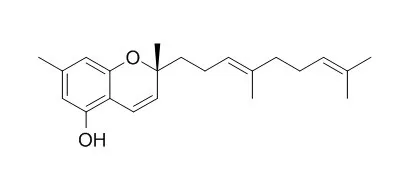
Eight grifolin derivatives, involving three new monomers, albatrelins A-C (1-3), three novel dimers (meroterpenoid pigments), albatrelins D-F (4-6), and two known ones, 6a,7,8,9,10,10a-hexahydro-3,6,9-trimethyl-6-(4-methyl-3-penten-1-yl)-1,9-epoxy-6H-dibenzo[b,d]pyran (7) and Confluentin (8), were isolated from Albatrellus ovinus. Their structures were established by extensive spectroscopic analysis. The absolute configurations of compounds 2-4 were determined as 9R by comparing their optical rotations with data reported in the literature.
CONCLUSIONS:
Albatrelin F (6) was isolated as a pair of C-2' tautomers with a ratio of 1.3:1. Confluentin (8) showed weak cytotoxicity against four human tumor cell lines, HL-60, SMMC-7712, A-549, and MCF-7, in vitro. | | Arch Pharm (Weinheim). 2003 Apr;336(2):119-26. | | Activities of prenylphenol derivatives from fruitbodies of Albatrellus spp. on the human and rat vanilloid receptor 1 (VR1) and characterisation of the novel natural product, confluentin.[Pubmed: 12761765] | Several prenylphenols from basidiocarps of European and Chinese Albatrellus spp., namely grifolin (1), neogrifolin (2), Confluentin (3), scutigeral (4), and albaconol (5) were investigated concerning their activities in test models for vanilloid receptor modulation.
METHODS AND RESULTS:
The isolation of these compounds from A. confluens and structure elucidation of the novel natural product Confluentin (3) are described. The effects of scutigeral and neogrifolin on vanilloid receptors were studied by means of electrophysiological methodology on rat dorsal root ganglion neurons as well as on recombinant cell lines expressing the rat VR1 receptor. Concurrently, the effects of compounds 1-5 on a reporter cell line expressing the human vanilloid receptor VR1 were measured.
CONCLUSIONS:
In contrast to previous studies reported in the literature, the results of these investigations suggest that fungal prenylphenols act as weak antagonists (activity in the microM range), rather than exhibiting agonistic activities. | | Planta Med. 2010 Feb;76(2):182-5. | | Antibacterial compounds from mushrooms I: a lanostane-type triterpene and prenylphenol derivatives from Jahnoporus hirtus and Albatrellus flettii and their activities against Bacillus cereus and Enterococcus faecalis.[Pubmed: 19644795] |
METHODS AND RESULTS:
Antibacterial bioassay-guided fractionation of two American mushroom species, Jahnoporus hirtus and Albatrellus flettii, led to the isolation and identification of their major antibacterial constituents: 3,11-dioxolanosta-8,24( Z)-diene-26-oic acid (1) from J. hirtus and Confluentin (2), grifolin (3), and neogrifolin (4) from A. flettii. Compound 1 is a new lanostane-type triterpene. All purified compounds were evaluated for their ability to inhibit the growth of Bacillus cereus and Enterococcus faecalis using standard MIC assays. Compounds 1- 4 demonstrated MIC values of 40, 20, 10, and 20 microg/mL, respectively, against B. cereus and MIC values of 32, 1.0, 0.5, and 0.5 microg/mL, respectively, against E. faecalis.
CONCLUSIONS:
Thus, one novel compound and three others were shown to possess antimicrobial activities against these gram-positive bacteria employed as surrogates for more virulent and dangerous pathogens. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)