| Description: |
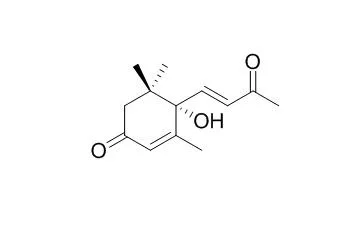
Dehydrovomifoliol could be a marker of Polish heather honey. Dehydrovomifoliol exhibits moderate acetylcholinesterase (AChE) inhibitory activities. Dehydrovomifoliol shows significant cytotoxic activities against three human cancer cell lines, namely, HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells, and the IC(50) values in the range 3.7-8.1 microM. |
| In vitro: |
| Chem Pharm Bull (Tokyo). 2010 Sep;58(9):1236-9. | | Anticholinesterase and antioxidant constituents from Gloiopeltis furcata.[Pubmed: 20823607] | Activity-directed isolation of the ethyl acetate, methylene chloride and n-hexane fractions of Gloiopeltis furcata resulted in the isolation of 18 compounds.
METHODS AND RESULTS:
Their structures were elucidated as 2-(3-hydroxy-5-oxotetrahydrofuran-3-yl)acetic acid (1), glutaric acid (2), succinic acid (3), nicotinic acid (4), (E)-4-hydroxyhex-2-enoic acid (5), cholesterol (6), 7-hydroxycholesterol (7), uridine (8), glycerol (9), 5-(hydroxymethyl)-2-methoxybenzene-1,3-diol (10), (5E,7E)-9-oxodeca-5,7-dienoic acid (11), (Z)-3-ethylidene-4-methylpyrrolidine-2,5-dione (12), Dehydrovomifoliol (13), loliolide (14), cholesteryl stearate (15), palmitic acid (16), cis-5,8,11,14,17-eicosapentaenoic acid (17) and alpha-linolenic acid (18) on the basis of spectroscopic and chemical evidences. Their anticholinesterase and antioxidant activities were evaluated via inhibitory activities on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) as well as scavenging activities on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and peroxynitrite (ONOO(-)).
CONCLUSIONS:
All isolated compounds (1-18) exhibited moderate AChE inhibitory activities with IC(50) values ranging from 1.14-12.50 microg/ml, whereas 1, 7, 9, 17, and 18 showed mild BChE inhibitory activities with IC(50) values ranging from 5.57-15.89 microg/ml.
Although most of the compounds isolated were lacking the scavenging activity on DPPH radical and ONOO(-), 5 and 10 showed good DPPH radical scavenging activity, and 5, 10, and 16 showed potent ONOO(-) scavenging activity. | | Chem Pharm Bull (Tokyo). 2009 Apr;57(4):408-10. | | Two new sesquiterpenoids from Solanum lyratum with cytotoxic activities.[Pubmed: 19336938] |
METHODS AND RESULTS:
Two new sesquiterpenoids, lyratol C (1) and lyratol D (2), together with two known sesquiterpenoids, Dehydrovomifoliol (3) and blumenol A (4), were isolated from the whole plant of Solanum lyratum. Their structures were established by spectroscopic analyses.
CONCLUSIONS:
In vitro, the four compounds showed significant cytotoxic activities against three human cancer cell lines, namely, HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells, and gave IC(50) values in the range 3.7-8.1 microM. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)