| Structure Identification: |
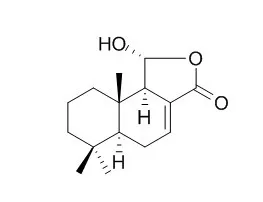
| Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):294-7. | | A monoclinic form of dendocarbin A: a borderline case of one-dimensional isostructural polymorphism.[Pubmed: 25836288] | The title compound, Dendocarbin A [systematic name: (1R,5aS,9aS,9bR)-1-hydroxy-6,6,9a-trimethyldodecahydronaphtho[1,2-c]furan-3-one], C15H22O3, is a sesquiterpene lactone isolated from Drimys winteri var chilensis.
METHODS AND RESULTS:
The monoclinic phase described herein displays an identical molecular structure to the orthorhombic phase that we reported previously [Paz Robles et al. (2014). Acta Cryst. C70, 1007-1010], while varying significantly in chain pitch, and can thus be considered as a borderline case of one-dimensional isostructural polymorphism. | | Acta Crystallogr C Struct Chem. 2014 Nov;70(Pt 11):1007-10. | | Dendocarbin A: a sesquiterpene lactone from Drimys winteri.[Pubmed: 25370095] | The natural compound Dendocarbin A, C15H22O3, is a sesquiterpene lactone isolated for the first time from Drimys winteri for var chilensis.
METHODS AND RESULTS:
The compound crystallizes in the orthorhombic space group P2₁2₁2₁ and its X-ray crystal structure confirmed the S/R character of the chiral centres at C-5/C-10 and C-9/C-11, respectively. The α-OH group at C-11 was found to be involved in intermolecular hydrogen bonding, defining chains along the <100> 2₁ screw axis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)