| In vitro: |
| Planta Medica, 2009, 75(07):757-762. | | Dynamic Residual Complexity of Natural Products by qHNMR: Solution Stability of Desmethylxanthohumol.[Reference: WebLink] | The use of chromatographic assays to assess the residual complexity of materials that are purified from natural sources by chromatographic means is, in a sense, a case of the fox watching the henhouse. Beside their static residual complexity, which is intrinsic to their metabolic origin, biologically active natural materials can also be involved in chemical reactions that lead to dynamic residual complexity.
METHODS AND RESULTS:
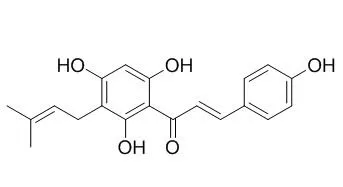
The present study examines the dynamics of the hop prenylphenol, Desmethylxanthohumol (DMX), by means of quantitative ¹H-NMR (qHNMR) in a setting that mimics in vitro and physiological conditions. The experiments provide a comprehensive, time-resolved, and mechanistic picture of the spontaneous isomerization of DMX into congeneric flavanones, including their ¹H/²D isotopomers. Formation of the potent phytoestrogen, 8-prenylnaringenin (8PN), suggests that measurable estrogenic activity even of high-purity DMX is an artifact. Together with previously established qHNMR assays including purity activity relationships (PARs), dynamic qHNMR assays complement important steps of the post-isolation evaluation of natural products.
CONCLUSIONS:
Thus, qHNMR allows assessment of several unexpected effects that potentially break the assumed linkage between a single chemical entity (SCE) and biological endpoints. | | Eur J Med Chem . 2017 Jan 5;125:335-345. | | Synthesis and antioxidant evaluation of desmethylxanthohumol analogs and their dimers[Pubmed: 27688188] | | Abstract
Four ring-closed analogs of natural prenylated chalcone Desmethylxanthohumol (1) and their dimers were synthesized from the commercially available 1-(2,4,6-trihydroxyphenyl)ethan-1-one in five and six linear steps, respectively. The structures of the eight new derivatives were confirmed using1H NMR, 13C NMR and HRMS. The antioxidant activity of the new chalcone derivatives were evaluated in a PC12 cell model of H2O2-induced oxidative damage. The SAR studies suggested that the catechol motif was essential for the antioxidant activity. Moreover, the dimers showed better antioxidant activity than their corresponding monomers did. Among them, compound 14d was the most potent and increased PC12 cell viability from 25% to 85%. Flow cytometric analysis showed that compound 14d, the most potent compound, decreased the apoptotic PC12 cell percentage and significantly reduced the LDH release and 8-OHdG generation but increased the GSH levels in H2O2-treated PC12 cells. Furthermore, compound 14d had a higher FRAP value than that of gallic acid. It also reduced the stable ABTS+ free radical with a lower EC50 than that of gallic acid.
Keywords: Antioxidant; Chalcone; Desmethylxanthohumol; Dimer; Synthesis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)