| Structure Identification: |
Tetrahedron,1975,31(4):305-9.
| | Constituents of coper-spurge seed (lathyridis seed)—II : Novel acid-catalyzed reactions of epoxylathyrol[Reference: WebLink] |
METHODS AND RESULTS:
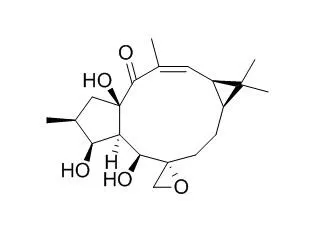
Reaction of Epoxylathyrol (1) with hydrochloric acid and other acids in THF led to simple fission of the epoxy ring and yielded the corresponding hydrin derivatives. When anhydrous methanol was used as the solvent, reaction of 1 with hydrochloric acid gave, as the major product, the novel macrocyclic trichloride (4) which has the jatrophane skeleton. Reaction with sulfuric acid afforded the transannular cyclization product (2) and methoxyhydrin (3). The structures of these compounds were deduced from spectral evidence. | | Phytochemical Analysis, 2001,12(4):255-62 | | HPLC-UV and HPLC-positive-ESI-MS analysis of the diterpenoid fraction from caper spurge (Euphorbia lathyris) seed oil[Reference: WebLink] |
METHODS AND RESULTS:
Caper spurge (Euphorbia lathyris L.) seed oil contains a series of diterpenoids known as Euphorbia factors, or L-factors, L1–L9. They are esters of several polyols (lathyrol, Epoxylathyrol, hydroxylathyrol and ingenol) and account for about 3–5% of the oil. The percentage of ingenol-based L-factors is very low, less than 5% of the diterpenoid fraction, but some of them (factors L5 and L6) are responsible for the irritant and co-carcinogenic activities of the oil. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)