| Kinase Assay: |
| Life Sci. 2013 Nov 4;93(18-19):655-63. | | A diterpenoid compound, excisanin A, inhibits the invasive behavior of breast cancer cells by modulating the integrin β1/FAK/PI3K/AKT/β-catenin signaling.[Pubmed: 24044886] | Excisanin A, a diterpenoid compound purified from Isodon macrocalyxin D, has anti-cancer properties with little toxicity. In this study, the anti-invasive effects of Excisanin A on breast cancer cells and its molecular mechanism of action were investigated.
METHODS AND RESULTS:
MTT, wound healing, transwell chamber and cell adhesion assays were utilized to investigate the effects of Excisanin A on MDA-MB-231 and SKBR3 cells. Western blotting, real-time PCR, RNA interference and luciferase reporter assays were employed to determine the molecular mechanism of action of Excisanin A.
Treating MDA-MB-231 and SKBR3 cells with 10-40μM Excisanin A significantly inhibited cell migration and invasion and suppressed the mRNA and protein levels of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in a dose-dependent manner. Excisanin A efficiently abolished integrin β1 expression and reduced the phosphorylation of the downstream kinases focal adhesion kinase (FAK) and Src. Excisanin A inhibited the phosphorylation of phosphoinositide 3-kinase (PI3K), AKT and glycogen synthase kinase 3 beta (GSK3β) and down-regulated β-catenin expression and the luciferase activity of the transcription factor LEF-1. Moreover, treating breast cancer cells with siRNA targeting integrin β1 inhibited cell invasion and migration.
CONCLUSIONS:
These results demonstrated that Excisanin A inhibited invasion by suppressing MMP-2 and MMP-9 expression; the integrin β1/FAK/PI3K/AKT/β-catenin signaling pathway was involved in this process. Therefore, Excisanin A might be a potential anti-metastatic chemotherapeutic agent for the treatment of breast cancer. | | Oncogene. 2006 Nov 9;25(53):7070-7. | | Acetylcholinesterase expression mediated by c-Jun-NH2-terminal kinase pathway during anticancer drug-induced apoptosis.[Pubmed: 16715131 ] |
It has been shown that acetylcholinesterase (AChE) expression was induced during apoptosis and the anti-sense oligonucleotides and siRNA of AChE may prevent apoptosis in various cell types. However, the mechanisms underlying AChE upregulation remain elusive. We demonstrated here that c-Jun NH2-terminal kinase (JNK) could mediate AChE expression.
METHODS AND RESULTS:
In this study, both etoposide and Excisanin A, two anticancer agents, induced apoptosis in colon cancer cell line SW620 as determined by Annexin V staining, the cleavage of caspase-3 and the proteolytic degradation of poly (ADP-ribose) polymerase (PARP). The results showed that both the agents upregulated AChE in SW620 cells. In the meantime, JNK was also activated and the expression and phosphorylation of c-Jun increased in SW620 cells exposed to the two agents. The induced AChE mRNA and protein expression could be blocked by SP600125, a specific inhibitor of SAPK/JNK, and small interfering RNA directed against JNK1/2. Transfection with adenovirus-mediated dominant negative c-Jun also blocked the upregulation of AChE expression.
CONCLUSIONS:
Together, these results suggest that AChE expression may be mediated by the activation of JNK pathway during apoptosis through a c-Jun-dependent mechanism. |
|
| Structure Identification: |
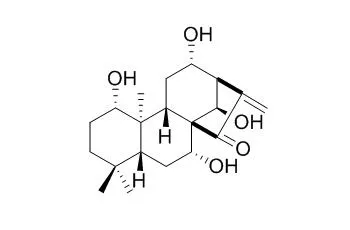
| Arch Pharm Res. 2008 Nov;31(11):1381-4. | | A new abietane diterpenoid from Isodon inflexus.[Pubmed: 19023532] |
METHODS AND RESULTS:
A new ent-abietane diterpenoid, 3alpha,6beta-dihydroxy-7,17-dioxo-ent-abieta-15(16)-ene (1), and three known ent-kaurane diterpenids, kamebacetal A (2), kamebakaurin (3), and Excisanin A (4), and a known triterpenoid, ursolic acid (5), were isolated from the aerial parts of Isodon inflexus. Their chemical structures were determined by extensive analysis of spectroscopic data including 1D-and 2D-NMR experiments.
CONCLUSIONS:
All isolates (1-5) were evaluated for their potential to inhibit LPS-induced nitric oxide production in RAW264.7 cells. Of these, compounds 1-4 inhibited the production of NO with IC(50) values ranging from 1.0 to 26.5 microM. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)