| Structure Identification: |
| J Org Chem. 2011 Apr 15;76(8):2694-700. | | Total synthesis of (-)-cocaine and (-)-ferruginine.[Pubmed: 21391709 ] |
METHODS AND RESULTS:
Total synthesis of tropane alkaloids (-)-cocaine and (-)-Ferruginine were accomplished in nine steps each and in 55% and 46% overall yields, respectively, starting from the known Betti base derivative (+)-(7aR,10R,12S)-10-(1H-benzotriazol-1-yl)-7a,8,9,10-tetrahydro-12-phenyl-12H-naphtho[1,2-e]pyrrolo[2,1-b][1,3]oxazine. In this novel route, RCM reaction and 1,3-dipolar cycloaddition were employed as key steps for the enantioselective construction of tropane skeleton and the regioselective introduction of 3-bromo-2-isoxazoline ring as masked cis-2,3-disubstituents.
CONCLUSIONS:
To obtain the desired precursor (2S,5R)-2-allyl-5-vinylpyrrolidine for RCM reaction, we developed a general and practical method for the preparation of enantiopure cis-2,5-disubstituted pyrrolidines bearing alkene- and/or alkyne-containing substituents. We also offered two highly efficient pathways for the conversion of the 3-bromo-2-isoxazoline ring into the desired cis-2,3-disubstituted groups in (-)-cocaine and (-)-Ferruginine. | | Australian Journal of Chemistry,1979, 32(11): 2537 -2543. | | Alkaloids of Darlingia ferruginea[Reference: WebLink] |
METHODS AND RESULTS:
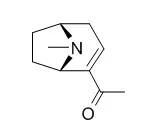
Darlingia ferruginea J. F. Bailey contains the new tropane alkaloids ferrugine (2) and 3α-benzoyloxy-2α-hydroxybenzyltropane (4) as well as darlingine (1) and Ferruginine (3), which also occur in D. darlingiana. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)