| In vivo: |
| Expert Opin Drug Saf. 2014 Jul;13(7):989-98. | | Overview and safety of fingolimod hydrochloride use in patients with multiple sclerosis.[Pubmed: 24935480] | Fingolimod (Gilenya®, FTY720) is an oral sphingosine-1-phosphate analogue that was approved by the FDA in 2010 for the treatment of relapsing forms of multiple sclerosis (MS).
METHODS AND RESULTS:
Fingolimod's mechanism of action is primarily related to lymphocyte sequestration in primary and secondary lymphoid tissues. Phase III trials demonstrated a reduction in annualized relapse rate and MRI progression in fingolimod-treated subjects compared with both placebo and IFN-β-treated subjects. Frequent adverse effects include fatigue, gastrointestinal disturbance, headache and upper respiratory tract infection. More serious, but rare, adverse events associated with fingolimod include atrioventricular block, symptomatic bradycardia, herpetic viral infections and macular edema.
We discuss the mechanism of action, pharmacokinetics, clinical efficacy and safety profile of fingolimod in patients with relapsing MS.
CONCLUSIONS:
Fingolimod is an effective treatment for relapsing MS and its oral route of administration may be preferred by some. Fingolimod is generally well tolerated but requires diligence in patient selection and monitoring. Additional information is needed regarding risk of infection, malignancy and rebound disease with long-term use of fingolimod. | | Chinese Journal of Tissue Engineering Research, 2014 ,18 (11):1712-7. | | Fingolimod hydrochloride suppresses inflammatory reaction of blood vessels after balloon injury of the carotid artery[Reference: WebLink] |
METHODS AND RESULTS:
Hematoxylin-eosin staining showed that the proliferation of blood vessel was remarkable in the balloon injury group, but attenuated in the drug intervention group. The appearance of blood vessels was normal in the blank control group and negative control group. Real-time fluorescent quantitative PCRrevealed that cyclooxygenase 2 and prostaglandin E2 mRNA expression levels were significantly lower in the drug intervention group than those in the balloon injury group at 7 days(P 0.05). Cyclooxygenase 2 and prostaglandin E2 mRNA expression levels were significantly higher in the balloon injury group and drug intervention group than those in the blank control group and negative control group at the same time point(P 0.05). Western blot assay results revealed that sphingosine1-phosphate receptor 1 expression was high in early stage of injury, and then reduced in late stage of injury. In particular, protein expression further decreased after drug intervention.
CONCLUSIONS:
Results indicated that Fingolimod hydrochloride suppressed inflammatory reaction of injured blood vessels and lessened the stenosis of injured blood vessels by regulating cyclooxygenase 2 and prostaglandin E2 mRNA expression using sphingosine1-phosphate receptor 1. |
|


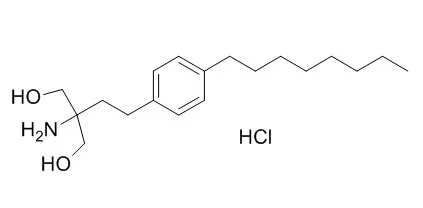



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)